Fig. 8.
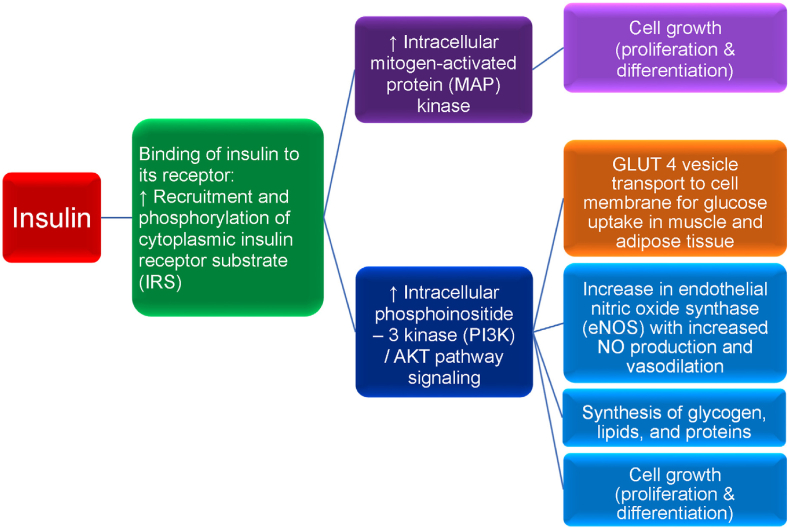
Insulin and insulin receptor functions. Diminished insulin activity can be due to an absolute or relative decrease in circulating insulin and/or impaired insulin signaling via a reduced number of insulin receptors and/or impaired post-receptor insulin signaling. Normoglycemia can be maintained in the early stages of insulin resistance by increased basal insulin (i.e., hyperinsulinemia), as often occurs in patients with obesity. However, over time, insulin secretion may no longer be sufficient to overcome insulin resistance, resulting in hyperglycemia. Obesity and the hyperglycemia of type 2 diabetes mellitus (T2DM) may result in a relative decrease in pancreatic insulin secretion, potentially due to elevations in leptin levels, as well as due to apoptosis with decreased pancreatic beta cell mass as the result of: (a) beta cell exhaustion/overload, (b) glucolipotoxicity, (c) increase in pro-inflammatory factors, and (d) decrease in anti-inflammatory factors (e.g., adiponectin). Insulin is a peptide hormone released by pancreatic beta cells in response to a rise in blood glucose (e.g., postprandial response to carbohydrate ingestion). Fructose, some amino acids, and fatty acids can also augment insulin release. Insulin binds to the extracellular alpha subunit portion of the transmembrane insulin cellular receptor of body tissues (e.g., liver, muscle, fat, brain). This activates a phosphorylation cascade involving transmembrane insulin receptor beta subunits that process tyrosine kinase activity, auto-phosphorylating insulin receptor tyrosines, and promoting the phosphorylation and activation of cytoplasmic insulin receptor substrate (IRS). Activated IRS stimulates intracellular mitogen-activated protein (MAP) kinase, which in turn, promotes cell growth (e.g., proliferation and differentiation of tissues, such as skeletal muscle cells and fat cells). While insulin mainly functions as a physiologic mitogenic facilitator, hyperinsulinemia may predispose to unregulated mitogenesis and cancer. Insulin-mediated phosphorylation of IRS also facilitates the phosphoinositide 3-kinase (PI3K)/AKT pathway (i.e., AKT is also known as protein kinase B), which is responsible for most of insulin's metabolic effects, such as the transport of glucose vesicle transporters (GLUT 4) to outer cellular membranes resulting in glucose uptake from the circulation into body tissues, thus lowering blood glucose. Insulin-dependent GLUT 4 is found in skeletal muscle and adipose tissue; insulin-independent GLUT 2 is found in the liver. Increased PI3K/AKT signaling also promotes (a) increase in endothelial nitric oxide synthase (eNOS) that facilitates increased nitric oxide production, increased vasodilation, and increased adipose tissue perfusion allowing for enhanced glucose and free fatty acids uptake in adipocytes for storage, (b) synthesis of glycogen, lipids, and proteins, and (c) cell growth (i.e., proliferation and differentiation). Dysregulation of the Ras/MAP kinase and/or PI3K/AKT pathways may adversely affect otherwise healthy cell growth (i.e., proliferation and differentiation), potentially leading to the development of human cancer [134,135], which has potential application regarding the increased risk of cancer among patient with obesity [136]. Among patients with obesity and T2DM, in addition to a reduced number of insulin receptors potentially as the result of impaired insulin receptor delivery to the cell surface due to endoplasmic reticulum stress, severe insulin resistance is mainly described as a post insulin receptor signaling defect, via disruption of the IR/IRS cascade. Specifically, obesity may result in adiposopathic increases in inflammatory factors (e.g., cytokines such as tumor necrosis factor and interleukins) and free fatty acids that may impair PI3K/AKT signaling while hyperinsulinemia continues to stimulate MAP kinase signaling, potentially contributing to post-receptor insulin resistance, prediabetes, T2DM, endothelial dysfunction, and/or cancer. (Adapted/copied with permission from: Bays HE, Obesity Pillars. 2023) [17].