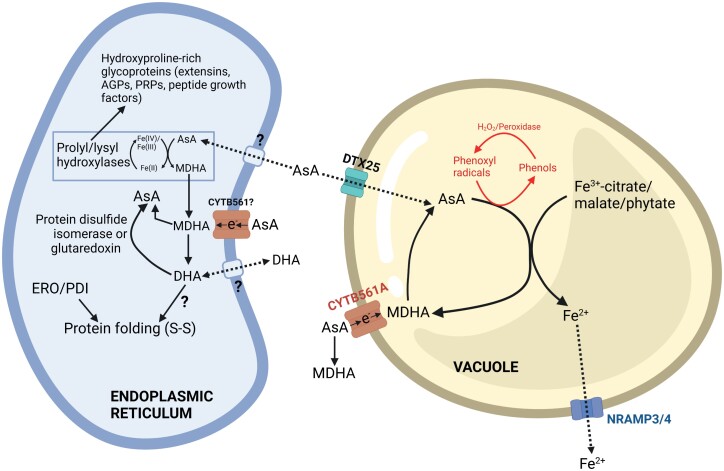
Fig. 6.
The role of ascorbate in iron-related processes. Ascorbate is required to prevent irreversible over-oxidation of Fe in 2-oxoglutarate-dependent dioxygenase (2-ODD) enzymes. 2-ODDs hydroxylating prolyl and lysyl residues of glycoproteins (e.g. prolyl 4-hydroxylase) are located in the ER. Inhibition or knockout of ER-localised prolyl 4-hydroxylases increases ascorbate concentration, suggesting that 2-ODD protection in the ER is a significant sink for ascorbate. Nothing is known about uptake of ascorbate or DHA, or the processes involved in reduction of MDHA or DHA in this compartment, but possible routes are shown. DHA could be reduced by GSH using PDI or glutaredoxin or MDHA could be reduced via cytc b5 mediated transmembrane electron transport. PDI is also involved in protein folding by disulfide bond formation, along with the H2O2-generating endoplasmic reticulum oxidoreductin (ERO). DHA could also facilitate disulfide bond formation. The recent identification of a tonoplast ascorbate transporter (DTX25) and transmembrane electron transporter (CYTB561A) provides a mechanism for moving ascorbate into the vacuole and regenerating MDHA. This system facilitates Fe mobilisation from vacuoles during seed germination and aids vacuolar H2O2 scavenging by type III peroxidases. Created with BioRender.com. Abbreviations: AGP, arabinogalactan protein; AsA, ascorbate; DHA, dehydroascorbate; ERO, endoplasmic reticulum oxidoreductin; P4H, prolyl 4-hydroxylase; MDHA, monodehydroascorbate; NRAMP, Natural resistance-associated macrophage protein; PDI, protein disulfide isomerase; PRP, proline-rich protein.