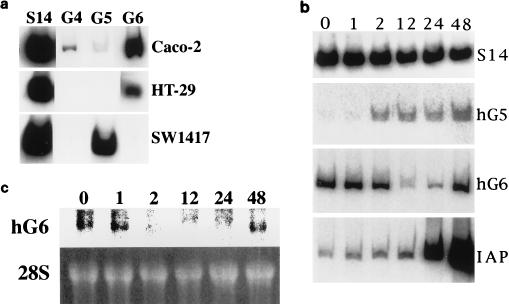
FIG. 7.
Changes in GATA factor transcript levels correlate with induction of terminal differentiation in human gut epithelium cell lines. (a) RT-PCR analysis was used to measure the relative levels of RNA encoding human GATA-4, -5, and -6 (G4, G5, and G6, respectively) in samples derived from uninduced and proliferating CaCo-2, HT-29, or SW1417 cells. The S14 gene encodes a relatively abundant rRNA protein message that does not change significantly in different samples and is used as a positive control for the RT reactions. PCR products were labeled by including trace radiolabeled nucleotides in the reaction. The products were detected after gel electrophoresis by autoradiography. (b) HT-29 cells were either uninduced (lane 0) or induced with 5 mM sodium butyrate, and RNA was harvested at various times (in hours) to measure relative transcript levels by semiquantitative RT-PCR analysis. A representative autoradiograph following gel electrophoresis of the PCR products is shown. The IAP gene is a terminal-differentiation marker that is induced to high levels by 48 h. The hGATA-5 gene is an early target for activation by sodium butyrate, while the hGATA-6 transcript levels decline initially during differentiation before recovering at later time points. Similar kinetics were consistently noted in multiple experiments. (c) The same RNA as that used for the RT-PCR analysis in panel b was analyzed for hGATA-6 mRNA in a Northern blotting experiment. Total RNA was electrophoresed, blotted, and hybridized to an hGATA-6 cDNA probe. As shown in the upper panel, the RNA levels decrease prior to reaccumulating by 48 h, confirming the RT-PCR results. The lower panel shows the ethidium bromide-stained 28S rRNA, demonstrating equal RNA loading for each lane.