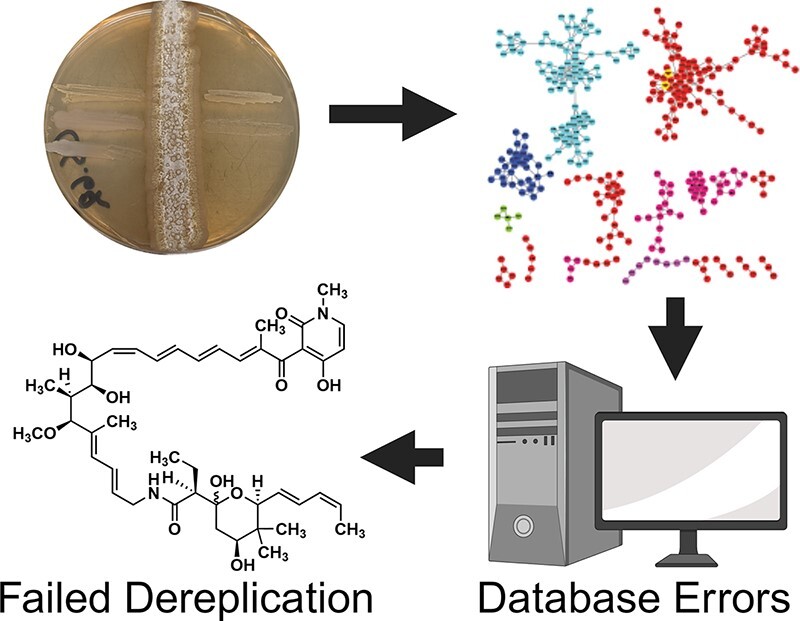
Abstract
Narrow-spectrum antibiotics are of great interest given their ability to spare the microbiome and decrease widespread antibiotic resistance compared to broad-spectrum antibiotics. Herein, we screened an in-house library of Actinobacteria strains for selective activity against Acinetobacter baumannii and successfully identified Streptomyces sp. CS-62 as a producer of a natural product with this valuable activity. Analysis of the cultures via high-resolution mass spectrometry and tandem mass spectrometry, followed by comparison with molecules in the Natural Product Atlas and the Global Natural Products Social Molecular Networking platform, suggested a novel natural product. Genome mining analysis initially supported the production of a novel kirromycin derivative. Isolation and structure elucidation via mass spectrometry and Nuclear Magnetic Resonance (NMR) analyses revealed that the active natural product was the known natural product factumycin, exposing omissions and errors in the consulted databases. While public databases are generally very useful for avoiding rediscovery of known molecules, rediscovery remains a problem due to public databases either being incomplete or having errors that result in failed dereplication. Overall, the work describes the ongoing problem of dereplication and the continued need for public database curation.
Keywords: Natural products, Biosynthetic gene cluster, Antibiotics, Dereplication
Graphical Abstract
Graphical Abstract.
Introduction
Actinobacteria have traditionally been a fantastic source of antimicrobials, including many clinically used antibiotics such as streptomycin, vancomycin, daptomycin, and rifampicin (Bérdy, 2012; Newman & Cragg, 2020). Given the antibiotic resistance crisis, there is great interest in identifying novel antibiotics, and Actinobacteria continue to be a great source of novel antibiotics (Genilloud, 2017). While the majority of antibiotics discovered to date are broad spectrum, species-selective molecules are increasingly sought after due to their reduced impact on the microbiome (Melander et al., 2018). Antibiotics selective for the ESKAPE pathogen Acinetobacter baumannii are particularly needed, given the high rates of antibiotic resistance as well as the high mortality rate of related infections (Appaneal et al., 2022; Howard et al., 2012). Interestingly, A. baumannii has a distinct membrane composition and biochemistry compared to other Gram-negative pathogens, suggesting that the discovery of an A. baumannii selective antibiotic may be possible (Melander et al., 2018). Only a handful of narrow-spectrum antimicrobials active against A. baumannii have been reported (Corey et al., 2017; Martin et al., 2019; Melander et al., 2018). For this reason, we sought to discover Actinobacteria natural products with selective activity against A. baumannii.
While natural products have been and continue to be a great source of antibiotics, the discovery of new natural products can be quite arduous. Dereplication is the process of identifying previously reported/known compounds within a sample. Modern genome mining analyses that enable identification of natural product biosynthetic gene clusters (BGCs), combined with high-resolution mass spectrometry and fragmentation methods, have led to much faster dereplication. (Henke & Kelleher, 2016) However, these methods depend on the availability of datasets deposited in libraries (Johnson & Lange, 2015; Sorokina & Steinbeck, 2020; Van Santen et al., 2019, 2021; Wang et al., 2016; Wishart et al., 2022). Specifically, dereplication of natural products is largely dependent on two types of databases: (1) genomic databases with natural product BGCs, and (2) metabolomic databases with mass spectra of natural products. For these databases to be useful for dereplication, they must both be accurate and up-to-date. This requires extensive manual curation. While other database submissions are required prior to publication (e.g., genome sequences to the NCBI and protein structures to the PDB), submission of most data important for natural products discovery are currently dependent on the motivation of the authors or volunteers. This data can be quite extensive including natural product structures to structural databases (e.g., Natural Product Atlas or COCONUT), BGCs to cluster databases (e.g., MiBIG), mass spectrometry data to mass spectrometry databases [e.g., Global Natural Products Social Molecular Networking (GNPS)], Nuclear Magnetic Resonance (NMR) data to NMR databases (e.g., NP-MRD or NMRxiv), and bioactivity data to bioactivity databases (e.g., ChEmBl, PubChem, or NPASS). Additional data types (e.g., micro-electron diffraction, microED) are also becoming more important as the field grows and evolves, and how this data will be handled must also be considered, including determining appropriate existing databases or generating new natural product-focused databases. While authors should deposit all of their relevant data in such databases, the fact remains that such depositions take time, and without external pressure to do so, many likely will not. Unfortunately, this is likely to remain a challenge until both journals and funding agencies require such depositions. While these depositions do take some time upfront, they ultimately will save the community much more time. Without deposition requirements, we will continue to have tedious and resource-wasting rediscoveries. Additionally, resources for the continued curation of these databases are also needed to ensure submissions are correct. Improving and expanding existing databases and establishing new databases will both benefit dereplication efforts and encourage artificial intelligence and machine learning efforts, which rely on the availability of good databases. In the long term, these computational methods are likely to lead to revolutionary findings.
Described herein, we isolated and screened new soil-dwelling Actinobacteria from Hanauma Bay, Hawaii, looking for selective activity against A. baumannii using a cross-streak approach. During this process, we discovered a novel Streptomyces strain that produces a compound with activity against A. baumannii. Metabolomics analyses and database comparisons suggested the discovery of a novel bioactive natural product. Comparative genomics analyses suggested the presence of a kirromycin-like BGC. However, isolation and NMR structure elucidation revealed that it was the known antibiotic, factumycin. This work highlights the importance of depositing BGCs and mass spectra into public databases and the continued need for community validation of data present in public databases.
Materials and Methods
See supplementary information.
Results and Discussion
Screening and Hit Identification
An in-house library of Actinobacteria was screened for their ability to selectively kill antibiotic-resistant strains of A. baumannii using a cross streak assay. Streptomyces sp. CS-62, a strain isolated from Hanauma Bay, Hawaii, was found to be active in this assay and was further explored (Figure S1). Streptomyces sp. CS-62 was grown in four media conditions: AGS, GUBC, MS, and ISP4. These supernatants were combined, desalted with a hydrophobic-lipophilic balance solid-phase extraction cartridge, and tested for biological activity at 100 µg/mL. A similar activity profile was observed to that in the cross-streak assay, with A. baumannii being the only Gram-negative bacteria having reproducible and robust sensitivity to the extract (Table S1). Additionally, the extract was nontoxic to the human lung cancer cell line A549 (<10% growth inhibition). Interestingly, the extract did show activity against S. aureus that was not initially observed in the cross-streak assay.
The metabolites present in the bioactive supernatant were then analyzed using liquid chromatography high-resolution mass spectrometry (LC-HRMS) and tandem mass spectrometry (MS2). This data was analyzed using GNPS and MolNetEnhancer and compared to the known molecules in the GNPS database (Fig. 1). While the masses of many known natural products were observed, the previously reported bioactivities of these natural products did not match the bioactivity we observed (Supplemental Excel File 1). We also compared the MS1 ions to m/z for natural products present in the Natural Product Atlas. Similarly, no known natural products were identified that were consistent with the bioactivity observed. As a result, we postulated that Streptomyces sp. CS-62 was potentially synthesizing a new natural compound.
Fig. 1.
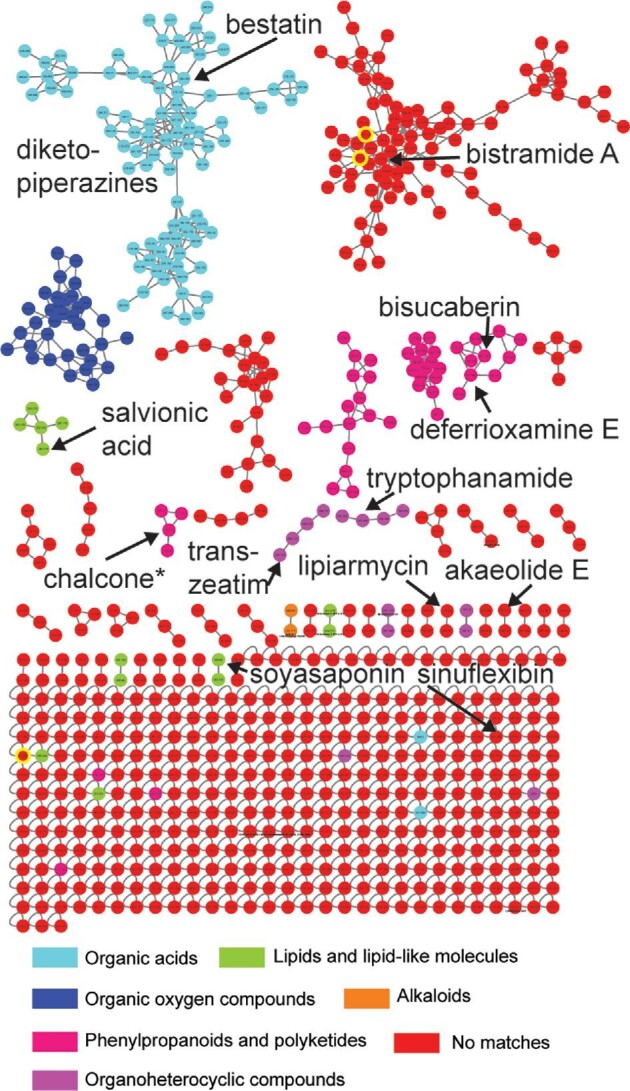
MolNET-enhanced Global Natural Products Social Molecular Networking (GNPS) network of Streptomyces sp. CS-62 crude extract from combined media. Colors represent predicted chemical superfamily. Molecules indicated were identified using GNPS.
We next determined that GUBC media was optimal for bioactive natural product isolation and then fractionated the supernatant to identify a semi-purified fraction with good activity. This fraction, along with fractions from media with varying levels of the bioactive natural product, were analyzed by LC-HRMS and MS2 (Table S1 and Figure S2). From this analysis, only one natural product, archazolid B, was proposed based on the GNPS analyses. The archazolids were originally isolated from myxobacteria and are known to inhibit mammalian VTPases at low nanomolar activity (Horstmann et al., 2011). However, to the best of our knowledge, archazolid B does not have specific antibiotic activity towards A. baumannii and thus is unlikely to be the natural product of interest in this study.
Genome Mining
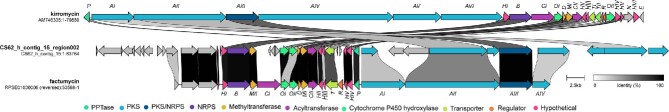
With the mass spectrometry data, the genome of Streptomyces sp. CS-62 was obtained using Illumina and Nanopore-integrated sequencing technologies. After assembly, the draft genome resulted in 31 contigs and 7.3 Mbp (Wick et al., 2017). The sequence was submitted to the Type (Strain) Genome Server and compared to the List of Prokaryotic Names with Standing in Nomenclature (Meier-Kolthoff & Göker, 2019; Meier-Kolthoff et al., 2022). Streptomyces sp. CS-62 was found to be a potential novel species with a d0 (dDDH) of 82.6% compared with the closest related strain, Streptomyces toxytricini. Analysis of the genome using antiSMASH 7.1.0 resulted in the identification of 46 BGCs (Table S2). Additionally, we determined the biosynthetic novelty index (González-Salazar et al., 2023) of the strain and found it to be 1129, suggesting it is highly likely to produce novel molecules. Two BGCs that had high similarities to BGCs that produce Gram-negative antibiotics were of particular interest: one with 69% identity to a BGC from Streptomyces collinus, which encodes for the known broad-spectrum antibiotic kirromycin, and another with 75% identity to streptothricin, a Gram-negative antibiotic (Morgan et al., 2023; Robertsen et al., 2018; Wolf et al., 1974). Given these similarities, we hypothesized that Streptomyces sp. CS62 could be producing a derivative of kirromycin or streptothricin with selective activity against A. baumannii.
Isolation and Structure Elucidation of the Bioactive Product Factumycin
Given the likelihood of a novel natural product, we performed bioactivity guided fractionation. Specifically, Streptomyces sp. CS-62 was cultivated on 10 L of GUBC agar. After freezing, the agar was squeezed to yield 8.5 L of aqueous supernatant. The supernatant was then fractionated using HLB resin. The 25% and 50% ACN fractions were further purified via preparatory HPLC. In all, this yielded 12.1 mg of a yellow solid of 95% purity (Figure S3A). With the molecule in hand, we first performed positive-mode HRMS analyses. m/z peaks were identified as 779.4482 and 801.4302 (Figure S3B). Assuming that these were the [M + H]+ and [M + Na]+ ions, respectively, we again manually searched the Natural Product Atlas and found no potential matching natural products within 5 ppm. 1H, 13C, and two-dimensional NMR spectra were obtained, and Small Molecule Accurate Recognition Technology (SMART) analyses were performed (Zhang et al., 2017). This resulted in a strong hit for mocimycin, a close derivative of kirromycin, suggesting that the molecule is an elfamycin (Figure S4). With this information, we revisited the kirromycin similar BGC in Streptomyces sp. CS-62 (Fig. 2 and Table 1). First, an NCBI BlastP search was performed with one of the polyketide synthases (PKSs) (cds89532_97800). Interestingly, a PKS from the factumycin BGC in Streptomyces sp. WAC5292 was identified to have 99% identity, suggesting that Streptomyces sp. CS-62 might be making the same molecule. This was further supported by CORASON analysis (Navarro-Muñoz et al., 2020; Quezada et al., 2017; Sélem-Mojica et al., 2019), which revealed that the BGC in Streptomyces sp. WAC5292 was one of the closest relatives (Figure S5). Streptomyces sp. WAC5292 is known to make the elfamycin-like molecule factumycin, which has selective antibiotic activity against A. baumannii (Charousová et al., 2018; Gullo et al., 1982; Thaker et al., 2012). A comparison of the m/z ions suggested that the molecule produced by Streptomyces sp. CS-62 was likely also factumycin, with NMR spectra further supporting this conclusion (Fig. 3, Figure S3 and Table S3). Additionally, the bioactivity closely resembles the previously reported selective activity against A. baumannii (Table S4) (Thaker et al., 2012). Interestingly, the CORASON analysis also revealed several other strains with highly similar BGCs, suggesting that these strains may also make factumycin or a closely related derivative (Figure S5).
Fig. 2.
Comparison of kirromycin, factumycin, and the Streptomyces sp. CS-62 biosynthetic gene clusters (BGCs). Clinker comparison of the kirromycin BGC from S. collinus sp. Tu 365, the factumycin BGC from Streptomyces sp. WAC5292, and the factumycin BGC from Streptomyces sp. CS62. Colors indicate type of gene.
Table 1.
Comparison of genes in factumycin cluster in Streptomyces CS62 to that in Streptomyces WAC5292 and the kirromycin cluster in S. collinus Tu 365
| Putative gene | CS62 gene location | WAC5292 (% ID) | S. collinus (%ID) |
|---|---|---|---|
| PKS | cds120830_123698 | 99% (facAIV) | 69% (kirAIV) |
| PKS | cds114552_120618 | 99% (facAIV) | 71% (kirAIV) |
| PKS | cds105045_109509 | 99% (facAIV) | 73% (kirAIV) |
| NRPS/PKS | cds99534_104193 | 99% (facAIII) | 69% (kirAIII) |
| PKS | cds97810_99538 | 98% (facAII) | 65% (kirAII) |
| PKS | cds89532_97800 | 99% (facAII) | 64% (kirAII) |
| PKS | cds79852_82153 | 98% (facAI) | 68% (kirAI) |
| 4'-phosphopantetheinyl transferase | cds78694_79498 | 99% (facP) | 61% (kirP) |
| Hypothetical | cds77680_78460 | 99% (facHIV) | 73% (kirHIV) |
| Hypothetical | cds77288_77684 | 99% (facHV) | 70% (kirHV) |
| MFS transporter | cds74652_75930 | 99% (facT) | 65% (kirT) |
| Hypothetical | cds74174_74597 | 68% (facHIII) | 49% (kirHIII) |
| Hypothetical | cds73630_74158 | 98% (facHII) | 60% (kirHII) |
| Acyltransferase | cds72151_73504 | 100% (facCII) | 65% (kirCII) |
| Methyltransferase | cds71157_72117 | 99% (facM) | 76% (kirM) |
| Aspartate-1-decarboxylase | cds70710_71130 | 99% (facD) | 78% (kirD) |
| Cytochrome P450 hydroxylase | cds69493_70699 | 99% (facOI) | 67% (kirOI) |
| Cytochrome P450 hydroxylase | cds68204_69494 | 99% (facOII) | 52% (kirOII) |
| Acyltransferase | cds67514_68165 | 99% (facCI) | 72% (kirCI) |
| Methyltransferase | cds63841_64843 | 99% (facMII) | none |
| NRPS | cds60723_63819 | 99% (facB) | 73% (kirB) |
| Hypothetical | cds59872_60727 | 99% (facHI) | 74% (kirHI) |
Note. %ID = percent identical.
Fig. 3.
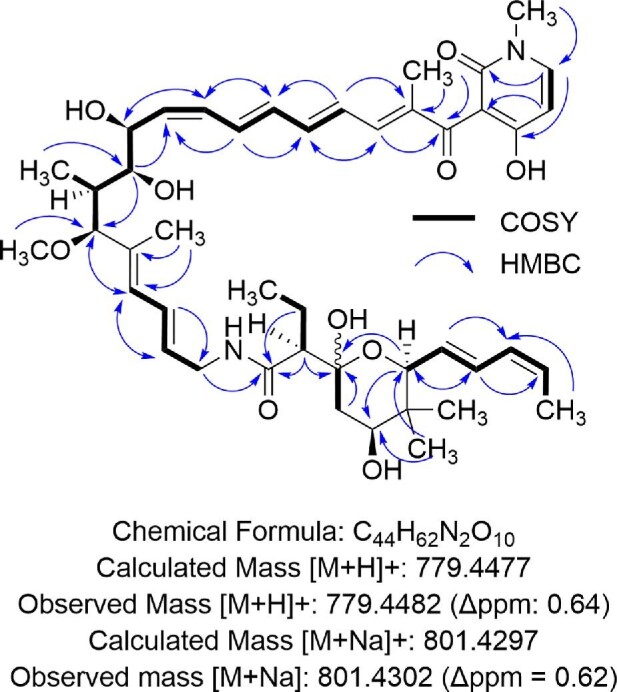
Structure of antibiotic natural product from Streptomyces sp. CS-62. Key NMR correlations along with the mass spectra confirm that the isolated natural product from Streptomyces sp. CS62 is factumycin.
Reasons for Dereplication Failure
Upon realizing that the Streptomyces sp. CS-62 molecule was factumycin, we analyzed the potential reasons that this was not dereplicated earlier. First, we utilized the structure search function in the GNPS database for both kirromycin and factumycin. For kirromycin, NCGC00384845 and NCGC00386087 were the only two compounds that came up as hits. Interestingly, these are the same molecules that SMART identified as the closest hits to the factumycin HSQC (Figure S4). However, when factumycin was used as the query, zero compounds were selected from the GNPS library, explaining the lack of elfamycins present in the molecular network. Additionally, when the mass spectrum of purified factumycin was used as the query, lauramine oxide was the only hit (Figure S6). Lauramine oxide is not related to the elfamycins and does not have a strong structural similarity to factumycin. In contrast to the GNPS library, the Natural Product Atlas database does have many elfamycins, including factumycin. Unfortunately, the structure, molecular weight, and exact mass of factumycin were incorrect, having an additional methyl group (Figure S7). Such mistakes are bound to happen in libraries such as the Natural Product Atlas because the structures are often manually entered. We have since submitted a correction to the Natural Product Atlas so that they can correct this error. Interestingly, COCONUT (Sorokina et al., 2021) has both the incorrect and correct structure in their database (Figure S8). The errors in both databases demonstrate how easily such errors propagate and demonstrate both the challenge of maintaining databases and the great need for continued manual curation.
Lesson Learned
Reflecting on our discoveries, we recognize the importance of high-quality structural databases in natural product workflows. Despite leveraging an array of computational tools in tandem with mass spectrometry techniques for dereplication, our study highlights their limitations. While improved structure and mass spectrometry databases would likely have allowed us to dereplicate factumycin more quickly, many challenges still exist. Expanding the scope of molecules submitted to the Natural Product Atlas and GNPS will improve identification. However, differences in instruments and experimental parameters used for mass spectrometry data acquisition and fragmentation will likely still result in many missed identifications. It is highly unlikely that the whole community will be able to settle on a single method, thus resulting in this being a long-term issue. Development of analyses techniques that can minimize these differences would be highly useful. Additionally, while mass spectrometry is a very useful tool, it does have limitations. Specifically, it often fails to identify compounds that ionize poorly. For this reason, it is important to focus on many different methods of dereplication as early as possible. The second method that is most often used is comparing BGCs in the organism to those in databases such as MiBIG. This is greatly enabling, allowing one to identify potential molecules or derivatives, but it does require having a genome sequence. Due to the decreasing costs of sequencing, this is becoming easier to attain. However, the method of sequencing highly affects one's ability to identify the BGCs. Given the large and often repetitive nature of many BGCs, long-read technologies are often necessary for proper identification. We observed this ourselves with our initial Illumina sequence not giving sufficient coverage for the discovery of the kirromycin-like (i.e., factumycin) BGC. Only after combining the Illumina results with nanopore MiniION long reads were we able to identify the BGC.
While both mass spectrometry and genome sequencing are often incorporated early into the dereplication process, other methods are likely needed to fully overcome this problem. NMR is a common strategy used for structure elucidation and is thus another great option to be included early on in the dereplication pipeline. For compounds containing relatively uncommon atoms (e.g., phosphorous, fluorine, or boron), this is a relatively simple process where crude extracts can be directly screened for these nuclei (Ju et al., 2015; Macho et al., 2022). Unfortunately, it is much more challenging for molecules that do not contain these relatively rare NMR active nuclei. 2D NMR spectra such as 1H-13C HSQC are incredibly useful for compound identification, with tools such as SMART and DP4 + greatly expediting structure elucidation (Franco et al., 2023; Grimblat et al., 2015; Hu & Qiu, 2023; Zhang et al., 2017). However, they generally do not work well on complex mixtures and are thus only applied late in the isolation process. More recently, NMR methods that can be applied with complex mixtures are being explored. LC-NMR-MS is a strategy that has previously been explored, but it is generally time-consuming due to the low abundance of molecules and requires special instrumentation that is not commonly available (Gebretsadik et al., 2021). More recently, strategies that combine 2D NMR with machine learning methods have been developed and hold great promise for aiding future dereplication efforts (Egan et al., 2021; Flores-Bocanegra et al., 2022; Hu & Qiu, 2023). Overall, dereplication continues to be a challenge in the natural products field. However, we believe that the continued development and improvement of the many libraries that are relevant to natural products discovery along with advances in dereplication strategies that can be applied to complex mixtures are likely to greatly mitigate this problem and reduce the chances of rediscoveries.
Supplementary Material
Acknowledgments
We acknowledge Bruce Cooper, Amber Jannasch, and the Bindley Bioscience Center, a core facility of the NIH-funded Indiana Clinical and Translational Sciences Institute for metabolomic analysis. We acknowledge John Harwood and the Purdue Interdepartmental NMR Facility for their assistance with the NMR analysis.
Contributor Information
Amir Y Alwali, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Diane Santos, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
César Aguilar, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Audrey Birch, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Lorena Rodriguez-Orduña, Centro de Biotecnología FEMSA, Tecnológico de Monterrey, Escuela de Ingeniería y Ciencias, 64849 Monterrey, México.
Carson B Roberts, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Ramya Modi, D epartment of Chemistry, Purdue University, West Lafayette, IN 47907, USA.
Cuauhtemoc Licona-Cassani, Centro de Biotecnología FEMSA, Tecnológico de Monterrey, Escuela de Ingeniería y Ciencias, 64849 Monterrey, México.
Elizabeth I Parkinson, Department of Chemistry, Purdue University, West Lafayette, IN 47907, USA; Department of Medicinal Chemistry and Molecular Pharmacology, Purdue University, West Lafayette, IN 47907, USA.
Funding
This work was supported by a grant from the National Institutes of Health (R35GM138002 to EIP).
Conflicts of Interest
The authors report no conflicts of interest.
Data Availability
All genome sequences associated with this paper have been deposited at NCBI under the BioProject accession number SAMN39682732. All the mzxML employed for the GNPS Networking are accessible as a publicly available MassIVE data set identified by MassIVE MSV000094200. Additionally, the MS2 spectra for the [M + Na]+ and the [M + H-3H20]+ have also been uploaded (GNPS Spectrum ID CCMSLIB00012194754 and CCMSLIB00012194758, respectively). All the NMRs employed in structure elucidation have been deposited at NP-MRD are available at https://depositions.np-mrd.org/request-data/0b0708cf-445c-4483-8c85-8b4b97d78dda. The factumycin BGC has also been suggested for inclusion in the MiBIG database.
References
- Appaneal H. J., Lopes V. V., LaPlante K. L., Caffrey A. R. (2022). Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrobial Agents and Chemotherapy, 66(3). 10.1128/aac.01975-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bérdy J. (2012). Thoughts and facts about antibiotics: Where we are now and where we are heading. The Journal of Antibiotics, 65(8), 385–395. 10.1038/ja.2012.27 [DOI] [PubMed] [Google Scholar]
- Charousová I., Medo J., Hleba L., Javoreková S. (2018). Streptomyces globosus DK15 and Streptomyces ederensis ST13 as new producers of factumycin and tetrangomycin antibiotics. Brazilian Journal of Microbiology, 49(4), 816–822. 10.1016/J.BJM.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey B. W., Thompson M. G., Hittle L. E., Jacobs A. C., Asafo-Adjei E. A., Huggins W. M., Melander R. J., Melander C., Ernst R. K., Zurawski D. V. (2017). 1,2,4-Triazolidine-3-thiones have specific activity against Acinetobacter baumannii among common nosocomial pathogens. ACS Infectious Diseases, 3(1), 62–71. 10.1021/ACSINFECDIS.6B00133/ASSET/IMAGES/LARGE/ID-2016-00133W_0006.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J. M., van Santen J. A., Liu D. Y., Linington R. G. (2021). Development of an NMR-based platform for the direct structural annotation of complex natural products mixtures. Journal of Natural Products, 84(4), 1044–1055. 10.1021/acs.jnatprod.0c01076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Bocanegra L., Al Subeh Z. Y., Egan J. M., El-Elimat T., Raja H. A., Burdette J. E., Pearce C. J., Linington R. G., Oberlies N. H. (2022). Dereplication of fungal metabolites by NMR-based compound networking using MADByTE. Journal of Natural Products, 85(3), 614–624. 10.1021/acs.jnatprod.1c00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco B. A., Luciano E. R., Sarotti A. M., Zanardi M. M. (2023). DP4+App: Finding the best balance between computational cost and predictive capacity in the structure elucidation process by DP4+. Factors analysis and automation. Journal of Natural Products, 86(10), 2360–2367. 10.1021/acs.jnatprod.3c00566 [DOI] [PubMed] [Google Scholar]
- Gebretsadik T., Linert W., Thomas M., Berhanu T., Frew R. (2021). LC–NMR for natural product analysis: A journey from an academic curiosity to a robust analytical tool. Sci, 3(1), 6. 10.3390/sci3010006 [DOI] [Google Scholar]
- Genilloud O. (2017). Actinomycetes: Still a source of novel antibiotics. Natural Product Reports, 34(10), 1203–1232. 10.1039/C7NP00026J [DOI] [PubMed] [Google Scholar]
- González-Salazar L. A., Quezada M., Rodríguez-Orduña L., Ramos-Aboites H., Capon R. J., Souza-Saldívar V., Barona-Gomez F., Licona-Cassani C. (2023). Biosynthetic novelty index reveals the metabolic potential of rare actinobacteria isolated from highly oligotrophic sediments. Microbial Genomics, 9(1), 000921. 10.1099/MGEN.0.000921/CITE/REFWORKS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimblat N., Zanardi M. M., Sarotti A. M. (2015). Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. The Journal of Organic Chemistry, 80(24), 12526–12534. 10.1021/acs.joc.5b02396 [DOI] [PubMed] [Google Scholar]
- Gullo V. P., Zimmerman S. B., Dewey R. S., Ray S., Cassidy P. J., Ōiwa R., Ōmura S. (1982). Factumycin, a new antibiotic (A40A): Fermentation, isolation and antibacterial spectrum. The Journal of Antibiotics, 35(12), 1705–1707. 10.7164/ANTIBIOTICS.35.1705 [DOI] [PubMed] [Google Scholar]
- Henke M. T., Kelleher N. L. (2016). Modern mass spectrometry for synthetic biology and structure-based discovery of natural products. Natural Product Reports, 33(8), 942–950. 10.1039/C6NP00024J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann N., Essig S., Bockelmann S., Wieczorek H., Huss M., Sasse F., Menche D. (2011). Archazolid A-15-O-β-D-glucopyranoside and iso-archazolid B: Potent V-ATPase inhibitory polyketides from the myxobacteria cystobacter violaceus and Archangium gephyra. Journal of Natural Products, 74(5), 1100–1105. 10.1021/NP200036V/SUPPL_FILE/NP200036V_SI_001.PDF [DOI] [PubMed] [Google Scholar]
- Howard A., O'Donoghue M., Feeney A., Sleator R. D. (2012). Acinetobacter baumannii. Virulence, 3(3), 5. 10.4161/VIRU.19700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Qiu M. (2023). Machine learning-assisted structure annotation of natural products based on MS and NMR data. Natural Product Reports, 40(11), 1735–1753. 10.1039/D3NP00025G [DOI] [PubMed] [Google Scholar]
- Johnson S. R., Lange B. M. (2015). Open-access metabolomics databases for natural product research: Present capabilities and future potential. Frontiers in Bioengineering and Biotechnology, 3(MAR), 130345. 10.3389/FBIOE.2015.00022/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju K.-S., Gao J., Doroghazi J. R., Wang K.-K. a., Thibodeaux C. J., Li S., Metzger E., Fudala J., Su J., Zhang J. K., Lee J., Cioni J. P., Evans B. S., Hirota R., Labeda D. P., van der Donk W. a., Metcalf W. W. (2015). Discovery of phosphonic acid natural products by mining the genomes of 10,000 actinomycetes. Proceedings of the National Academy of Sciences of the United States of America, 112(39), 12175–12180. 10.1073/pnas.1500873112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho J. M., Blue R. M., Lee H.-W., MacMillan J. B. (2022). Boron NMR as a method to screen natural product libraries for B-containing compounds. Organic Letters, 24(17), 3161–3166. 10.1021/acs.orglett.2c00885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. E., Melander R. J., Brackett C. M., Scott A. J., Chandler C. E., Nguyen C. M., Minrovic B. M., Harrill S. E., Ernst R. K., Manoil C., Melander C. (2019). Small molecule potentiation of gram-positive selective antibiotics against Acinetobacter baumannii. ACS Infectious Diseases, 5(7), 1223–1230. 10.1021/ACSINFECDIS.9B00067/ASSET/IMAGES/LARGE/ID-2019-00067D_0006.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Kolthoff J. P., Carbasse J. S., Peinado-Olarte R. L., Göker M. (2022). TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Research, 50(D1), D801–D807. 10.1093/NAR/GKAB902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Kolthoff J. P., Göker M. (2019). TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nature Communications, 10(1), 2182. 10.1038/S41467-019-10210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander R. J., Zurawski D. V., Melander C. (2018). Narrow-spectrum antibacterial agents. MedChemComm, 9(1), 12–21. 10.1039/C7MD00528H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C. E., Kang Y. S., Green A. B., Smith K. P., Dowgiallo M. G., Miller B. C., Chiaraviglio L., Truelson K. A., Zulauf K. E., Rodriguez S., Kang A. D., Manetsch R., Yu E. W., Kirby J. E. (2023). Streptothricin F is a bactericidal antibiotic effective against highly drug-resistant gram-negative bacteria that interacts with the 30S subunit of the 70S ribosome. PLoS Biology, 21(5), e3002091. 10.1371/JOURNAL.PBIO.3002091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Muñoz J. C., Selem-Mojica N., Mullowney M. W., Kautsar S. A., Tryon J. H., Parkinson E. I., De Los Santos E. L. C., Yeong M., Cruz-Morales P., Abubucker S., Roeters A., Lokhorst W., Fernandez-Guerra A., Cappelini L. T. D., Goering A. W., Thomson R. J., Metcalf W. W., Kelleher N. L., Barona-Gomez F., Medema M. H. (2020). A computational framework to explore large-scale biosynthetic diversity. Nature Chemical Biology, 16(1), 60–68. 10.1038/s41589-019-0400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. Journal of Natural Products, 83(3), 770–803. 10.1021/acs.jnatprod.9b01285 [DOI] [PubMed] [Google Scholar]
- Quezada M., Licona-Cassani C., Cruz-Morales P., Salim A. A., Marcellin E., Capon R. J., Barona-Gómez F. (2017). Diverse cone-snail species harbor closely related Streptomyces species with conserved chemical and genetic profiles, including polycyclic tetramic acid macrolactams. Frontiers in Microbiology, 8(NOV), 271214. 10.3389/FMICB.2017.02305/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen H. L., Musiol-Kroll E. M., Ding L., Laiple K. J., Hofeditz T., Wohlleben W., Lee S. Y., Grond S., Weber T. (2018). Filling the gaps in the kirromycin biosynthesis: Deciphering the role of genes involved in ethylmalonyl-CoA supply and tailoring reactions. Scientific Reports 2018 8:1, 8(1), 1–9. 10.1038/s41598-018-21507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sélem-Mojica N., Aguilar C., Gutiéerrez-García K., Martínez-Guerrero C. E., Barona-Gómez F. (2019). Evomining reveals the origin and fate of natural product biosynthetic enzymes. Microbial Genomics, 5(12), e000260. 10.1099/MGEN.0.000260/CITE/REFWORKS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokina M., Merseburger P., Rajan K., Yirik M. A., Steinbeck C. (2021). COCONUT online: Collection of Open Natural Products database. Journal of Cheminformatics, 13(1), 1–13. 10.1186/S13321-020-00478-9/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokina M., Steinbeck C. (2020). Review on natural products databases: Where to find data in 2020. Journal of Cheminformatics, 2020 12:1, 12(1), 1–51. 10.1186/S13321-020-00424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker M. N., García M., Koteva K., Waglechner N., Sorensen D., Medina R., Wright G. D. (2012). Biosynthetic gene cluster and antimicrobial activity of the elfamycin antibiotic factumycin. MedChemComm, 3(8), 1020–1026. 10.1039/C2MD20038D [DOI] [Google Scholar]
- Van Santen J. A., Jacob G., Singh A. L., Aniebok V., Balunas M. J., Bunsko D., Neto F. C., Castaño-Espriu L., Chang C., Clark T. N., Cleary Little J. L., Delgadillo D. A., Dorrestein P. C., Duncan K. R., Egan J. M., Galey M. M., Haeckl F. P. J., Hua A., Hughes A. H., Linington R. G. (2019). The Natural Products Atlas: An open access knowledge base for microbial natural products discovery. ACS Central Science, 5(11), 1824–1833. 10.1021/acscentsci.9b00806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Santen J. A., Kautsar S. A., Medema M. H., Linington R. G. (2021). Microbial natural product databases: Moving forward in the multi-omics era. Natural Product Reports, 38(1), 264–278. 10.1039/D0NP00053A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Carver J. J., Phelan V. V., Sanchez L. M., Garg N., Peng Y., Nguyen D. D., Watrous J., Kapono C. A., Luzzatto-Knaan T., Porto C., Bouslimani A., Melnik A. V., Meehan M. J., Liu W.-T., Crüsemann M., Boudreau P. D., Esquenazi E., Sandoval-Calderón M., Bandeira N. (2016). Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nature Biotechnology, 34(8), 828–837. 10.1038/nbt.3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick R. R., Judd L. M., Gorrie C. L., Holt K. E. (2017). Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Computational Biology, 13(6), e1005595. 10.1371/JOURNAL.PCBI.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D. S., Sayeeda Z., Budinski Z., Guo A. C., Lee B. L., Berjanskii M., Rout M., Peters H., Dizon R., Mah R., Torres-Calzada C., Hiebert-Giesbrecht M., Varshavi D., Varshavi D., Oler E., Allen D., Cao X., Gautam V., Maras A., Cort J. R. (2022). NP-MRD: The Natural Products Magnetic Resonance Database. Nucleic Acids Research, 50(D1), D665–D677. 10.1093/NAR/GKAB1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Chinali G., Parmeggiani A. (1974). Kirromycin, an inhibitor of protein biosynthesis that acts on elongation factor tu. Proceedings of the National Academy of Sciences, 71(12), 4910–4914. 10.1073/PNAS.71.12.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Idelbayev Y., Roberts N., Tao Y., Nannapaneni Y., Duggan B. M., Min J., Lin E. C., Gerwick E. C., Cottrell G. W., Gerwick W. H. (2017). Small molecule accurate recognition technology (SMART) to enhance natural products research. Scientific Reports 7:1, 7(1), 1–17. 10.1038/s41598-017-13923-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genome sequences associated with this paper have been deposited at NCBI under the BioProject accession number SAMN39682732. All the mzxML employed for the GNPS Networking are accessible as a publicly available MassIVE data set identified by MassIVE MSV000094200. Additionally, the MS2 spectra for the [M + Na]+ and the [M + H-3H20]+ have also been uploaded (GNPS Spectrum ID CCMSLIB00012194754 and CCMSLIB00012194758, respectively). All the NMRs employed in structure elucidation have been deposited at NP-MRD are available at https://depositions.np-mrd.org/request-data/0b0708cf-445c-4483-8c85-8b4b97d78dda. The factumycin BGC has also been suggested for inclusion in the MiBIG database.