Abstract
Background
This study evaluated the safety and pharmacokinetics (PK) of oral ONC201 administered twice-weekly on consecutive days (D1D2) in pediatric patients with newly diagnosed DIPG and/or recurrent/refractory H3 K27M glioma.
Methods
This phase 1 dose-escalation and expansion study included pediatric patients with H3 K27M-mutant glioma and/or DIPG following ≥1 line of therapy (NCT03416530). ONC201 was administered D1D2 at 3 dose levels (DLs; −1, 1, and 2). The actual administered dose within DLs was dependent on weight. Safety was assessed in all DLs; PK analysis was conducted in DL2. Patients receiving once-weekly ONC201 (D1) served as a PK comparator.
Results
Twelve patients received D1D2 ONC201 (DL1, n = 3; DL1, n = 3; DL2, n = 6); no dose-limiting toxicities or grade ≥3 treatment-related adverse events occurred. PK analyses at DL2 (D1-250 mg, n = 3; D1-625 mg, n = 3; D1D2-250 mg, n = 2; D1D2-625 mg, n = 2) demonstrated variability in Cmax, AUC0–24, and AUC0–48, with comparable exposures across weight groups. No accumulation occurred with D1D2 dosing; the majority of ONC201 cleared before administration of the second dose. Cmax was variable between groups but did not appear to increase with D1D2 dosing. AUC0–48 was greater with D1D2 than once-weekly.
Conclusions
ONC201 given D1D2 was well tolerated at all DLs and associated with greater AUC0–48.
Keywords: ONC201, dordaviprone, H3 K27M mutation, dose optimization
Key Points.
ONC201 administered twice-weekly on consecutive days (D1D2) did not result in DLTs or treatment-related adverse events leading to discontinuation.
As expected, D1D2 ONC201 administration resulted in greater exposure but did not lead to accumulation.
Importance of the Study.
ONC201 has previously demonstrated promising efficacy and safety in patients with H3 K27M-mutated diffuse glioma, a disease associated with exceptionally poor survival. Prior trials evaluated once-weekly or once every 3 week administration of ONC201; this dose optimization study evaluated the safety and pharmacokinetics of twice-weekly ONC201 administered on 2 consecutive days in pediatric patients with H3 K27M-mutant glioma. Safety was comparable to once-weekly administration and dose escalation proceeded to the highest dosing level without any dose-limiting toxicities. Pharmacokinetic analyses demonstrated no accumulation with twice-weekly ONC201 administration. Dose optimization remains an important consideration in clinical trials of novel compounds; this analysis suggests that twice-weekly administration of ONC201 has comparable safety to once-weekly administration and is currently being evaluated in a large phase 3 trial (ACTION).
Diffuse intrinsic pontine glioma (DIPG) is a childhood central nervous system (CNS) cancer for which there currently is no cure. Despite the use of radiation therapy, median progression-free survival is 6 months (95%CI, 5.6–6.4) and overall survival (OS) is 11 months (10.5–11.5).1 Significant surgical resection is not an option given the location of the tumor2 and chemotherapy has not shown any benefit to date.3,4 Molecular analysis of autopsy and biopsy tissue of DIPG have found that up to 70% of DIPGs and 60% of adult thalamic gliomas contain the H3 K27M mutation.5,6 In 2016, the World Health Organization classified DIPG and other diffuse gliomas with the H3 K27M mutation to be a new grade 4 glioma termed diffuse midline glioma, H3 K27M-mutant. In 2021, the definition was expanded to diffuse midline glioma, H3 K27-altered to additionally include diffuse midline gliomas with EZHIP overexpression, which can also cause loss of H3 K27me3, similar to the H3 K27M mutation.7,8 New therapeutic options are desperately needed.
ONC201, the first in the imipridone class of agents, is a small, blood–brain barrier penetrant antagonist of dopamine receptor D2 and an agonist of caseinolytic peptidase P (ClpP). The compound induces the TRAIL pathway in a p53-independent manner, the integrated stress response, and other effects that subsequently lead to tumor cell death.9–13
In the first-in-human trial of ONC201 in adults with refractory solid tumors, ONC201 was administered once every 3 weeks at doses ranging from 125 mg to 625 mg to identify the recommended phase 2 dose (RP2D); this schedule was selected based upon in vitro efficacy, pharmacokinetics (PK), and pharmacodynamic data.14,15 In that study, the maximum planned ONC201 dose of 625 mg every 3 weeks was reached without dose-limiting toxicity.14 Subsequent studies in adult cancer patients found that ONC201 administered once-weekly at 625 mg ONC201 was well tolerated and resulted in increased systemic immunostimulatory activity compared to the every 3 week schedule.16
In a phase 2 study of ONC201 administered once every 3 weeks in adults with molecularly unselected recurrent glioblastoma, one patient who incidentally harbored the H3 K27M mutation experienced a durable objective response.17,18 Subsequently, a phase 1 study of ONC201 in pediatric patients with H3 K27M-mutant DMG and/or DIPG was initiated in January 2018. This open-label, dose-escalation, and dose-expansion trial administered ONC201 as an intact capsule once-weekly following at least one prior course of therapy, which included irradiation. The drug was well tolerated with no ≥grade 3 adverse events attributed to ONC201 and the RP2D was the adult equivalent RP2D of 625 mg, scaled by weight.19 Subsequently, the trial was expanded to include additional arms, including a dose escalation arm of patients who received ONC201 twice-weekly on 2 consecutive days.
In the present report, the day 1 and day 2 dosing regimen was selected to provide prolonged ONC201 exposures over some time of at least 48 h. Per preclinical data, sustained ONC201 exposure was associated with the death of newly diagnosed and recurrent glioblastoma cells.20 The present study evaluated twice-weekly dosing of ONC201 for the first time in patients to assess the applicability of preclinical findings to the clinical setting. Here we describe the RP2D, PK, and safety outcomes of pediatric patients who received ONC201 administered on this schedule.
Materials and Methods
Patient Population and Study Design
ONC014 (NCT03416530) was a phase 1 multi-arm, open-label, dose-escalation study in pediatric patients with DIPG or H3 K27M-mutant glioma. The protocol was reviewed and approved by individual institutions IRB; written informed consent was obtained for all patients. Eligibility included patients with glioma with the H3 K27M mutation shown by immunohistochemistry or sequencing in a Clinical Laboratory Improvement Amendment-certified laboratory or agreement to postmortem biopsy. Because of the high frequency of H3 K27M mutation in DIPG,6 tissue confirmation of the mutation was not required at study entry for patients with DIPG. At study entry, patients were aged 2 to <19 years, had a Karnofsky/Lansky performance status (KPS/LPS) score of ≥50, adequate organ function, and a minimum weight of 10 kg. Patients must have completed at least one line of prior therapy, with appropriate washout from anti-cancer therapies before initiating ONC201 treatment; this included 5 half-lives from any investigational agent, 4 weeks from cytotoxic therapy (except 23 days for temozolomide and 6 weeks from nitrosoureas), 6 weeks from antibodies, or 4 weeks (or five half-lives, whichever is shorter) from other anti-tumor therapies. For patients who received radiotherapy, patients were at least 2 weeks from the completion of local radiotherapy (re-irradiation for progressive disease or upfront radiation at initial diagnosis). Evidence of tumor progression/recurrence was not required. Exclusion criteria included patients with diffuse leptomeningeal disease or cerebral spinal fluid dissemination based on investigator assessment. The primary endpoint of the overarching trial was the determination of the RP2D; secondary outcomes included safety, PK, and progression-free survival.
Intact ONC201 capsules were administered orally twice-weekly on 2 consecutive days; cycles were 21 days, (ie, each cycle consisting of 6 doses). Dose escalation was performed using a 3 + 3 design with a dose expansion for 6 patients at dose level 2 if feasible. Dose cohorts were sequentially designated as −1, 1 and 2 with the target dose per cohort based on the body weight adjusted adult target of 375, 500, and 625 mg, respectively (Supplementary Table 1). For patients ≤ 55 kg, the dose was allometrically scaled using a power model assuming an average adult weight of 70 kg and an exponent of ¾.21 Doses were rounded to the nearest 125 mg capsule. Dose by body weight was 3.1–4.2 mg/kg in dose level −1, 6.3–8.3 mg/kg in dose level 1, and 12.5–16.7 mg/kg in dose level 2. Body weight was assessed at the start of each 21-day cycle and rounded to the nearest 5 kg interval, with consideration of adjusted weight for patients on steroids.
The RP2D was defined as the maximum tolerated dose or maximum administered dose (MAD). Dose limiting toxicity (DLT) was defined as a drug-related adverse event (AE) or abnormal laboratory value that occurred in the first cycle of treatment, met criteria for DLT in Supplementary Table 2, and was assessed as unrelated to disease, disease progression, inter-current illness, or concomitant medications, and was judged by the investigator to be “possibly related,” “probably related” or “definitely related” to ONC201.
Treatment beyond disease progression (including with bevacizumab and/or re-irradiation) was permitted if it was considered in the best interest of the patient and no significant adverse events attributed to the ONC201 were present.
PK data were assessed for patients in all dose levels. For the primary analysis, patients treated with twice-weekly ONC201 at dose level 2 were compared to patients treated with once-weekly ONC201 at dose level 2 in a separate arm of the same clinical trial.19 For summary analyses of Tmax, Cmax, AUC0-24, and AUC0-48, as well as the plasma–concentration time-curve, twice-weekly patients were compared against patients who received once-weekly ONC201 at dose level 2 and had a matching administered dose (mg). For analyses by body weight, patients treated at dose level 2 in the once- and twice-weekly regimens were included, regardless of the actual administered dose (mg).
Sparse plasma PK sampling was collected from all patients on cycle 1 day 1 (C1D1) before ONC201 administration and 0.5, 2, 4, and 24 h postdose, with the 24-h collection occurring treatment on C1D2. For patients receiving twice-weekly dosing, additional samples were collected on cycle 1 day 2 (C1D2), and sparse plasma sampling was collected at 0.5, 2, and 4 h post treatment. In all patients, additional collections occurred on C1D3 (24 h sample from day 2), C1D8 (predose), C1D15 (predose), and C2D1 (predose); in subsequent cycles, plasma was collected prior to treatment on the first day of even numbered cycles (Supplementary Figure 1). The samples were analyzed using 50 ml of K2EDTA plasma by a validated LC/MS-MS method with positive ESI-MRM mode that had a linear range of 1–500 ng/ml. Deuterated ONC201 was used as an internal standard. ONC201 PK parameters were calculated using the noncompartmental analysis.
Study Evaluations
History and physical exam were performed weekly during the first cycle and prior to the start of each subsequent cycle. Laboratory studies including complete blood count (CBC), comprehensive metabolic evaluation, magnesium, phosphorus, uric acid, lactate dehydrogenase (LDH), amylase, and pregnancy test for individuals of child-bearing potential were performed prior to each cycle. A standard 12-lead electrocardiogram (ECG) was performed prior to the start of ONC201 and repeated during therapy as deemed necessary. AEs were assessed following initiation of therapy and continued until 30 days following the last dose of ONC201 using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5. The cutoff date for analyses was February, 16th, 2023.
Results
Patient Characteristics
Among 12 patients receiving twice-weekly ONC201, all patients had previously received radiation. Median age was 9 years (range, 4–18 years) and 9 patients (75.0%) had a KPS/LPS ≥ 80. The most common tumor location was the pons (n = 8, 66.7%), followed by the thalamus (n = 2, 16.7%), and midbrain and spinal cord (each n = 1, 8.3%; Table 1). H3 K27M status was available in eight patients, of which 5 (41.7%) were detected by immunohistochemistry and 3 (25.0%) by next generation sequencing. The median time from initial diagnosis was 5.9 months (range, 3.0–13.1) and the median time from prior radiation to ONC201 initiation was 60.5 days (range, 29–290). The median duration of treatment was 3.2 months (range, 1.2–28.3). Eight patients (66.7%) were treated prior to recurrence. Three patients (25.0%) were treated following a second course of radiation therapy. Dose level assignment and actual administered dose are summarized in Supplementary Table 3.
Table 1.
Demographic and Baseline Characteristics
| Parameter | Patients (n = 12) |
|---|---|
| Age, median (range), years | 9 (4–18) |
| Female sex, n (%) | 8 (66.7) |
| Race, n (%) | |
| White | 9 (75.0) |
| Unknown | 2 (16.7) |
| Other | 1 (8.3) |
| Body weight, median (range), kg | 34.7 (15.3–63.7) |
| KPS/LPS, median (range) | |
| <80 | 3 (25.0) |
| ≥80 | 9 (75.0) |
| Time from diagnosis, median (range), month | 5.9 (3.0–13.1) |
| Primary tumor location, n (%) | |
| Pons | 8 (66.7) |
| Thalamus | 2 (16.7) |
| Midbrain | 1 (8.3) |
| Spinal cord | 1 (8.3) |
| Diagnosis, n (%) | |
| Diffuse midline glioma, H3 K27M-mutant | 8 (66.7) |
| DIPG (biopsied) | 4 (33.3) |
| Non-DIPG (biopsied) | 4 (33.3) |
| DIPG (nonbiopsied) | 4 (33.3) |
| Multifocal disease, n (%) | |
| Yes | 2 (16.7) |
| No | 10 (83.3) |
| Disease status, n (%) | |
| Post radiation, not recurrent | 8 (66.7) |
| Recurrent | 4 (33.3) |
| H3 K27M mutation method, n (%) | |
| Immunohistochemistry | 5 (41.7) |
| Next-generation sequencing | 3 (25.0) |
| H3.3 K27M mutation | 3 (25.0) |
| H3.1 K27M mutation | 0 |
| Unknown | 4 (33.3) |
| Number of recurrences, n (%) | |
| 0 | 8 (66.7) |
| 1 | 2 (16.7) |
| ≥2 | 2 (16.7) |
| Time from prior radiation, median (range), days | 60.5 (29.0–290) |
| Re-irradiation, n (%) | 3 (25.0) |
| Dexamethasone daily dose, median (range), mg | 0.2 (0–4) |
Safety
The trial proceeded through dose level 2 without any DLTs. Three patients were treated on dose levels −1 and 1, and a total of 6 patients were treated at dose level 2 (3 initially and 3 in dose expansion). All patients in the twice-weekly treatment regimen experienced at least one treatment-emergent AE (TEAE). Grade 3 or higher TEAEs occurred in 5 (41.7%) patients (dose level −1, n = 2; dose level 1, n = 1; dose level 2, n = 2), the most common of which was gait disturbance (n = 3, 25.0%; Table 2). No grade 3 or higher TEAEs were determined to be related to treatment.
Table 2.
Summary of Safety
| Patients (n = 12) | ||||
|---|---|---|---|---|
| All causality | Treatment-related | |||
| All Grades | Grade ≥ 3 | All Grades | Grade ≥ 3 | |
| Patients with any TEAE, n (%)a | 12 (100.0) | 5 (41.7) | 8 (66.7) | 0 |
| Fatigue | 8 (66.7) | 1 (8.3) | 2 (16.7) | 0 |
| Headache | 7 (58.3) | 0 | 1 (8.3) | 0 |
| Vomiting | 6 (50.0) | 1 (8.3) | 0 | 0 |
| Decreased appetite | 4 (33.3) | 0 | 0 | 0 |
| Gait disturbance | 4 (33.3) | 3 (25.0) | 0 | 0 |
| Hyperglycemia | 4 (33.3) | 0 | 0 | 0 |
| Hyponatremia | 4 (33.3) | 0 | 0 | 0 |
| Nausea | 4 (33.3) | 0 | 1 (8.3) | 0 |
| Anemia | 3 (25.0) | 0 | 1 (8.3) | 0 |
| Blood lactate dehydrogenase increased | 3 (25.0) | 0 | 0 | 0 |
| Diarrhea | 3 (25.0) | 0 | 1 (8.3) | 0 |
| Muscular weakness | 3 (25.0) | 0 | 0 | 0 |
| Pyrexia | 3 (25.0) | 0 | 0 | 0 |
| Alanine aminotransferase increased | 2 (16.7) | 0 | 1 (8.3) | 0 |
| Dehydration | 2 (16.7) | 1 (8.3) | 0 | 0 |
| Dysarthria | 2 (16.7) | 0 | 0 | 0 |
| Dysphagia | 2 (16.7) | 1 (8.3) | 0 | 0 |
| Facial nerve disorder | 2 (16.7) | 0 | 0 | 0 |
| Hemiparesis | 2 (16.7) | 1 (8.3) | 0 | 0 |
| Hypercalcemia | 2 (16.7) | 0 | 0 | 0 |
| Hypermagnesemia | 2 (16.7) | 0 | 0 | 0 |
| Hypertension | 2 (16.7) | 1 (8.3) | 1 (8.3) | 0 |
| IIIrd nerve disorder | 2 (16.7) | 0 | 0 | 0 |
| Lymphocyte count decreased | 2 (16.7) | 0 | 1 (8.3) | 0 |
| Vith nerve disorder | 2 (16.7) | 0 | 0 | 0 |
| Accessory nerve disorder | 1 (8.3) | 0 | 0 | 0 |
| Amylase increased | 1 (8.3) | 0 | 1 (8.3) | 0 |
| Anal incontinence | 1 (8.3) | 0 | 0 | 0 |
| Anxiety | 1 (8.3) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 1 (8.3) | 0 | 0 | 0 |
| Aspiration | 1 (8.3) | 0 | 0 | 0 |
| Ataxia | 1 (8.3) | 0 | 1 (8.3) | 0 |
| Chills | 1 (8.3) | 0 | 0 | 0 |
| Confusional state | 1 (8.3) | 0 | 0 | 0 |
| Conjunctival irritation | 1 (8.3) | 0 | 0 | 0 |
| Conjunctivitis | 1 (8.3) | 0 | 0 | 0 |
| Constipation | 1 (8.3) | 0 | 0 | 0 |
| Cushingoid | 1 (8.3) | 0 | 0 | 0 |
| Dysuria | 1 (8.3) | 0 | 0 | 0 |
| Face edema | 1 (8.3) | 0 | 1 (8.3) | 0 |
| Facial asymmetry | 1 (8.3) | 0 | 0 | 0 |
| Glossopharyngeal nerve disorder | 1 (8.3) | 1 (8.3) | 0 | 0 |
| Hydrocephalus | 1 (8.3) | 1 (8.3) | 0 | 0 |
| Hyperkalaemia | 1 (8.3) | 0 | 0 | 0 |
| Hyperphosphatemia | 1 (8.3) | 0 | 1 (8.3) | 0 |
| Hypocalcemia | 1 (8.3) | 0 | 0 | 0 |
| Hypoglossal nerve disorder | 1 (8.3) | 0 | 0 | 0 |
| Hypomagnesaemia | 1 (8.3) | 0 | 0 | 0 |
| Hypophosphatemia | 1 (8.3) | 0 | 0 | 0 |
| Insomnia | 1 (8.3) | 0 | 1 (8.3) | 0 |
| Intraocular pressure increased | 1 (8.3) | 0 | 0 | 0 |
| Musculoskeletal pain | 1 (8.3) | 0 | 0 | 0 |
| Neuropathy peripheral | 1 (8.3) | 0 | 0 | 0 |
| Edema peripheral | 1 (8.3) | 0 | 0 | 0 |
| Optic nerve disorder | 1 (8.3) | 0 | 0 | 0 |
| Renal colic | 1 (8.3) | 0 | 0 | 0 |
| Sinus tachycardia | 1 (8.3) | 0 | 0 | 0 |
| Skin infection | 1 (8.3) | 0 | 0 | 0 |
| Somnolence | 1 (8.3) | 0 | 0 | 0 |
| Stomatitis | 1 (8.3) | 0 | 0 | 0 |
| Urinary hesitation | 1 (8.3) | 0 | 0 | 0 |
| Urinary incontinence | 1 (8.3) | 0 | 0 | 0 |
| Urinary tract infection | 1 (8.3) | 0 | 0 | 0 |
| Visual impairment | 1 (8.3) | 1 (8.3) | 0 | 0 |
| Weight increased | 1 (8.3) | 0 | 1 (8.3) | 0 |
| White blood cell count decreased | 1 (8.3) | 0 | 0 | 0 |
aSome patients experienced more than one TEAE.s
Treatment-related TEAEs (TR-TEAEs) occurred in 8 patients (66.7%); all TR-TEAEs were grade 1–2 in severity and included fatigue (n = 2, 16.7%), headache, nausea, anemia, diarrhea, alanine aminotransferase increase, hypertension, lymphocyte count decreased, amylase increased, ataxia, face edema, hypophosphatemia, insomnia, and weight increased (each n = 1, 8;3%).
Four patients experienced serious AEs that were not considered treatment-related by the investigator, including dysphagia (n = 2, 16.7%), vomiting, fatigue, gait disturbance, pyrexia, headache, and hydrocephalus (each n = 1, 8.3%). No treatment-related SAEs occurred. No AEs resulted in a dose reduction or DLT, regardless of causality. One patient discontinued ONC201 due to AEs considered unlikely to be related to treatment (8.3%). After the onset of these AEs, an MRI confirmed disease progression, which was felt by the investigator to be the cause of the AEs.
Pharmacokinetic Assessment
Among 6 patients treated with twice-weekly ONC201 at dose level 2, data for PK analysis was available for 4 patients (250 mg, n = 2; 625 mg, n = 2). To evaluate differences in the pharmacokinetics of once- and twice-weekly dosing, patients who received ONC201 once-weekly at dose level 2 with matching administered doses (250 mg, n = 3; 625 mg, n = 3) were used as a comparator.
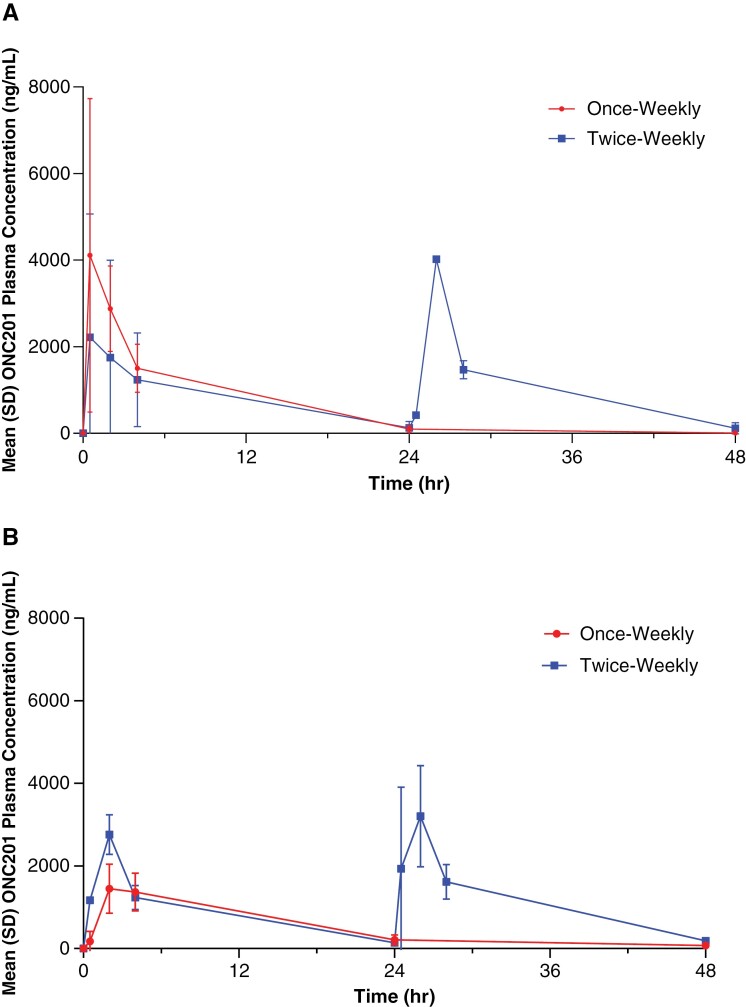
When comparing like doses within dose level 2, the median time of maximum concentration (Tmax) was 14.25 (250 mg) and 25.25 h (625 mg) for twice-weekly patients, compared to 0.5 h (250 mg) and 2 h (625 mg) for patients receiving once-weekly ONC201 (Figure 1; Supplementary Table 3).
Figure 1.
ONC201 Plasma concentration–time curves for patients at dose level 2 receiving 250 mg (A) or 625 mg (B) ONC201 once or twice-weekly.a Red, once-weekly; blue, twice-weekly on consecutive days.
The mean Cmax was 2670 and 3700 ng/ml following twice-weekly administration of 250 (n = 2) and 625 mg ONC201 (n = 2), respectively. For the same administered doses in the once-weekly arm, the mean Cmax was 4743 and 1787 ng/ml (Supplementary Table 4). Across cohorts, Cmax was variable. Noticeably, Cmax did not appear to increase with successive twice-weekly doses. As expected, AUC0-48 was greater in patients receiving twice-weekly ONC201 compared to the once-weekly weekly regimen; this trend was consistent across doses (250 mg and 625 mg).
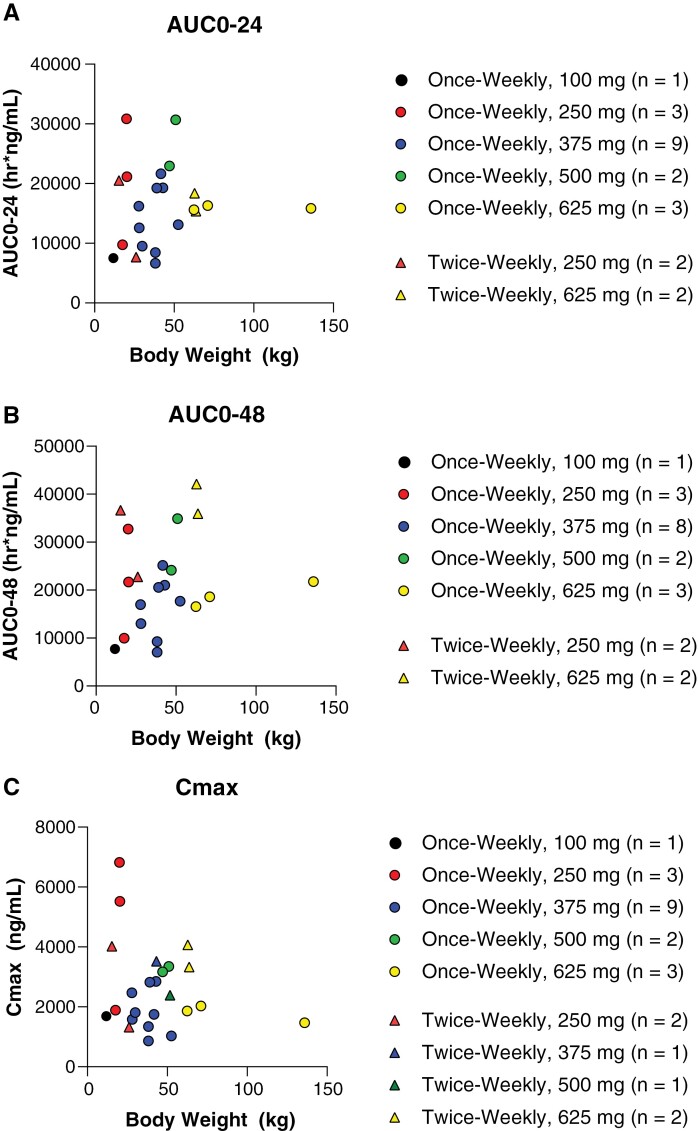
Following noncompartmental analysis and reporting of PK parameters for direct comparison of doses within dose level 2, an exploratory analysis was conducted to evaluate the effect of body weight on exposure parameters AUC0-24, AUC0-48, and Cmax, which were plotted by weight and identified by dose (Figure 2). As dose level 2 administered ONC201 in the range of 12.5–16.7 mg/kg, all patients in dose level 2 with available data were included, regardless of the actual administered dose. The analysis for AUC0-24 and AUC0-48 contained 4 patients from the twice-weekly dosing arm (250 mg, n = 2; 625 mg, n = 2). The analysis for Cmax contained 6 patients from the twice-weekly dosing arm (250 mg, n = 2; 375 mg, n = 1; 500 mg, n = 1; 625 mg, n = 2), which was inclusive of 2 patients excluded from AUC analyses due to lack of Day 2 predose timepoints, which were required for AUC calculation. For AUC0-24, AUC0-48, and Cmax body weight analyses, 18 patients from the once-weekly dosing arm (125 mg, n = 1; 250 mg, n = 3; 375 mg, n = 9, 500 mg, n = 2; 625 mg, n = 3) served as a comparator. There was variability within doses for all exposure parameters. As identified in the noncompartmental analysis, AUC0-48 exposures trended higher in twice-weekly dosing; this trend was maintained across increasing body weight. Cmax analysis was variable, displaying the greatest exposures following 250 mg in lower body weight patients (~25 kg).
Figure 2.
ONC201 AUC0-24 (A), AUC0-48 (B), and Cmax (C) by body weight among patients treated at dose level 2.
Discussion
Among pediatric patients with H3 K27M glioma, twice-weekly dosing on consecutive days regimen was well tolerated and did not result in DLTs or TR-TEAEs leading to discontinuation, which was comparable to previously reported once-weekly ONC201 administration.19 Furthermore, TR-TEAEs observed with twice-weekly on consecutive day dosing were limited to grade 1–2 severity, of which the most common was fatigue.
Relative to once-weekly dosing, twice-weekly ONC201 administration resulted in a greater exposure over 48 h after dosing (AUC0-48); this was not unanticipated since the twice-weekly regimen provides twice the weekly dose. Mean Cmax in the twice-weekly population was 2670 (250 mg) and 3700 (625 mg) ng/ml. This exceeded the target therapeutic concentration of 1000 ng/ml, which was established based on the approximate EC50s for ClpP and DRD2 identified in preclinical research.11,12,17,20 Exposure varied within doses given to the same body weight and across increased body weight, however, ONC201 remained well tolerated in lower body weight patients. The small population size (twice-weekly n = 2; once-weekly, n = 3, once-weekly) in the PK analysis as well as the sparse PK sampling schedule contributed to the PK variability observed in the study. The pronounced variability observed for Cmax in particular may be attributed to the lack of PK sampling in between 0.5 and 2 h postdose, the range of the once-weekly Tmax.
Previously, an integrated analysis was conducted to evaluate the safety and efficacy of ONC201 monotherapy in adult and pediatric patients with recurrent H3 K27M-mutant diffuse midline glioma; most patients were treated with once-weekly (n = 49), except 1 patient who was treated once every 3 weeks (n = 1). The overall response rate was 20.0% (95%CI, 10.0–33.7) by RANO-HGG criteria. The median time to response was 8.3 months (range, 1.9–15.9) and responses were durable, with a median duration of response of 11.2 months. Median OS was 13.7 months (95%CI, 8.0–20.3). Similar to the present analysis, treatment was generally well tolerated, with no discontinuations or deaths due to TRAE, and no grade 4 TRAEs reported.22 In the present analysis, the mixed population of patients with various tumor sites and recurrent/nonrecurrent disease precluded the interpretation of treatment efficacy using this regimen. A comprehensive analysis of the efficacy of twice-weekly dosing is therefore warranted. To this end, the phase 3 ACTION trial is evaluating the efficacy and safety of both once- and twice-weekly ONC201 in pediatric and adult patients with H3 K27M-mutant diffuse glioma (NCT05580562; Arrillaga-Romany, 2023).
A limitation of this analysis is the sparse sampling within the limited PK dataset. It is not known if the trends highlighted in this small cross-cohort comparison will carry over into the larger patient population. Additional data and analysis will be needed to confirm trends.
Dose optimization remains an important consideration when developing novel cancer therapies; the present analysis demonstrates comparable safety of once-weekly and twice-weekly on consecutive days of treatment regimens, as well as an increase in exposure. Given the preclinical data suggesting the added benefit of prolonged ONC201 exposure,20 the twice-weekly on consecutive days regimen of ONC201 merits additional investigation to determine its utility in clinical use. The ongoing phase 3 clinical trial for ONC201 will expand upon the present findings of this clinical evaluation, including administration of crushed capsules in food/liquid, and increased population size to allow better interpretation of the data and utility of the twice-weekly on consecutive days regimen in the clinic.
Supplementary Material
Acknowledgments
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc. Medical writing support was provided by Meghan Sullivan, PhD, CMPP, an employee of Chimerix, Inc.
Contributor Information
Yazmin Odia, Department of Neuro-Oncology, Miami Cancer Institute at Baptist Health South Florida, Miami, Florida, USA.
Carl Koschmann, Department of Pediatrics, University of Michigan, Ann Arbor, Michigan, USA.
Nicholas A Vitanza, The Ben Towne Center for Childhood Cancer Research, Seattle Children’s Research Institute, Seattle, Washington, USA; Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Washington, Seattle, Washington, USA.
Peter de Blank, Department of Pediatric Neuro-Oncology, University of Cincinnati Medical Center, Cincinnati, Ohio, USA.
Dolly Aguilera, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University, Atlanta, Georgia, USA.
Jeffrey Allen, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Doured Daghistani, Department of Neuro-Oncology, Miami Cancer Institute at Baptist Health South Florida, Miami, Florida, USA.
Matthew Hall, Department of Neuro-Oncology, Miami Cancer Institute at Baptist Health South Florida, Miami, Florida, USA; Department of Radiation Oncology, Nicklaus Children’s Hospital, Miami, Florida, USA.
Ziad Khatib, Department of Radiation Oncology, Nicklaus Children’s Hospital, Miami, Florida, USA.
Cassie Kline, Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Division of Oncology, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
Tobey MacDonald, Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University, Atlanta, Georgia, USA.
Sabine Mueller, Department of Clinical Pediatrics and Neurosurgery, University of California, San Francisco; California, USA.
Shamia L Faison, Certara Inc., Princeton, New Jersey, USA.
Joshua E Allen, Chimerix, Inc, Durham, North Carolina, USA.
Odin J Naderer, Chimerix, Inc, Durham, North Carolina, USA.
Samuel C Ramage, Chimerix, Inc, Durham, North Carolina, USA.
Rohinton S Tarapore, Chimerix, Inc, Durham, North Carolina, USA.
Susan Lynne McGovern, Department of Pediatric Neuro-Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Soumen Khatua, Department of Pediatric Neuro-Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA; Division of Pediatric Hematology/Oncology, Mayo Clinic, Rochester, Minnesota, USA.
Wafik Zaky, Department of Pediatric Neuro-Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sharon L Gardner, Department of Pediatrics, New York University Grossman School of Medicine, New York, New York, USA.
Conflict of interest statement
Y.O. has received clinical trial support from Chimerix, Inc. C.Kl. has received clinical trial funding and support from Chimerix, Inc. S.M. has received clinical trial funding and support from Chimerix, Inc. S.L.F. is a contract employee of Chimerix, Inc. J.E.A. is an employee of Chimerix, Inc, owns Chimerix, Inc. stock, and owns licenses with milestones and royalties related to ONC201 and other imipridones. O.J.N., S.C.R., and R.S.T. are employees of Chimerix, Inc. and own Chimerix, Inc. stock. Susan Lynne McGovern has served on advisory boards for Chimerix, Inc. C.Ko., N.A.V., P.de.B., D.A., J.A., D.D., M.H., Z.K., T.McD., S.K., W.Z., and S.G. have no conflict of interests to declare.
Supplement sponsorship
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc.
Funding
This study was funded by Chimerix, Inc.; additional clinical trial funding was provided by The Making Headway Foundation, Inc.
Author Contributions
Study design: J.E.A., R.S.T., S.L.G. Data collection: Y.O., C.Ko., N.A.V., P.d., D.A., J.A., D.D., M.H., Z.K., C.Kl., T.M., S.M., S.L.M., S.K., W.Z., S.L.G. Statistical analyses: S.L.F., O.J.N. Manuscript preparation: S.L.G., S.L.F., J.E.A., O.J.N., S.C.R., R.S.T. Manuscript review: all authors: Y.O., C.Ko., N.A.V., P.D., D.A., J.A., D.D., M.H., Z.K., C.Kl., T.M., S.M., S.L.F., J.E.A., O.J.N., S.C.R., R.S.T., S.L.M., S.K., W.Z., S.L.G.
Data Availability
Patient-level data collected as part of this study is not available for analysis by independent researchers. Aggregated data will be provided via publicly accessible databases as required by law. For more information, please contact clinicaltrials@chimerix.com.
References
- 1. Veldhuijzen van Zanten SEM, Baugh J, Chaney B, et al. Members of the SIOPE DIPG Network. Development of the SIOPE DIPG network, registry and imaging repository: a collaborative effort to optimize research into a rare and lethal disease. J Neurooncol. 2017;132(2):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johung TB, Monje M.. Diffuse intrinsic pontine glioma: new pathophysiological insights and emerging therapeutic targets. Curr Neuropharmacol. 2017;15(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 2011;13(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abe H, Natsumeda M, Kanemaru Y, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas and MGMT silencing to temozolomide sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo). 2018;58(7):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng J, Hao S, Pan C, et al. The H33 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol. 2015;46(11):1626–1632. [DOI] [PubMed] [Google Scholar]
- 6. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H33 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171–ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Free RB, Cuoco CA, Xie B, et al. Pharmacological characterization of the imipridone anticancer drug ONC201 reveals a negative allosteric mechanism of action at the D2 dopamine receptor. Mol Pharmacol. 2021;100(4):372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Madhukar NS, Khade PK, Huang L, et al. A Bayesian machine learning approach for drug target identification using diverse data types. Nat Commun. 5221;10(1):2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721–737.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graves PR, Aponte-Collazo LJ, Fennell EMJ, et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol. 2019;14(5):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stein MN, Bertino JR, Kaufman HL, et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23(15):4163–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen JE, Crowder RN, El-Deiry WS.. First-in-class small molecule ONC201 induces DR5 and cell death in tumor but not normal cells to provide a wide therapeutic index as an anti-cancer agent. PLoS One. 2015;10(11):e0143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stein MN, Malhotra J, Tarapore RS, et al. Safety and enhanced immunostimulatory activity of the DRD2 antagonist ONC201 in advanced solid tumor patients with weekly oral administration. J ImmunoTher Cancer. 2019;7(136). https://pubmed.ncbi.nlm.nih.gov/31118108/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrillaga-Romany I, Chi AS, Allen JE, et al. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arrillaga-Romany I, Odia Y, Prabhu VV, et al. Biological activity of weekly ONC201 in adult recurrent glioblastoma patients. Neuro Oncol 2020;22(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardner SL, Tarapore RS, Allen J, et al. Phase I dose escalation and expansion trial of single agent ONC201 in pediatric diffuse midline gliomas following radiotherapy. Neurooncol Adv. 2022;4(1):vdac143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prabhu VV, Lulla AR, Madhukar NS, et al. Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PLoS One. 2017;12(8):e0180541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barbour AM, Fossler MJ, Barrett J.. Practical considerations for dose selection in pediatric patients to ensure target exposure requirements. AAPS J. 2014;16(4):749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arrillaga-Romany I, Kurz S, Tarapore R, et al. LTBK-05 clinical efficacy of ONC201 in recurrent H3 K27M-mutant diffuse midline glioma patients. Neuro-Oncol. 2021;23(S6):vi230–vi230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Patient-level data collected as part of this study is not available for analysis by independent researchers. Aggregated data will be provided via publicly accessible databases as required by law. For more information, please contact clinicaltrials@chimerix.com.