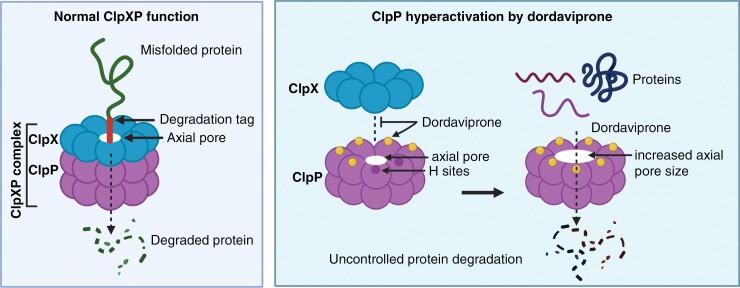
Figure 3.
Hyperactivation of ClpP by dordaviprone. During respiration, misfolded proteins are identified by a degradation tag and degraded by the ClpXP complex, passing through the axial pore. Dordaviprone replaces ClpX and binds ClpP, in H sites, removing protein selectivity and hyperactivating its protease activity. Additionally, dordaviprone increases axial pore size, resulting in more efficient degradation of mitochondrial respiratory complex proteins, regardless of their folding.