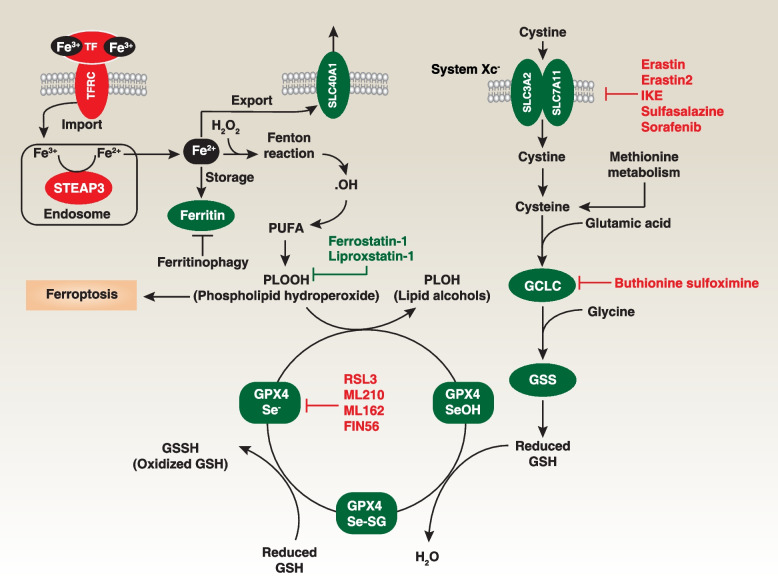
Fig. 3.
GPX4-dependent pathway in ferroptosis. The GPX4-dependent antiferroptotic pathway is associated with classical ferroptosis, a form of cell death driven by iron-dependent lipid peroxidation of PUFA. In this process, Fe3+ is transported into the intracellular space through binding to TF (transferrin) and its receptor TFRC. Within endosomes, STEAP3 converts intracellular Fe3+ to Fe2+, which can catalyze the production of .OH through the Fenton reaction, leading to lipid peroxidation. Conversely, storing Fe2+ in ferritin or exporting iron into the extracellular space via SLC40A1 can inhibit ferroptosis. Furthermore, the degradation of ferritin through ferritinophagy increases free Fe2+ levels, thereby promoting ferroptosis. GPX4 plays a pivotal role in this pathway by catalyzing the reduction of toxic PLOOH to non-toxic PLOH. The activity of GPX4 is tightly regulated by GSH (glutathione) and Se (selenium). Intracellular GSH levels are primarily controlled by system xc--mediated cystine uptake and subsequent cysteine transformation for GSH synthesis. In addition to the system xc-, methionine metabolism serves as an additional intracellular source of cysteine for the synthesis of GSH. The radical-trapping antioxidants ferrostatin-1 and liproxstatin-1 are the most used ferroptosis inhibitors