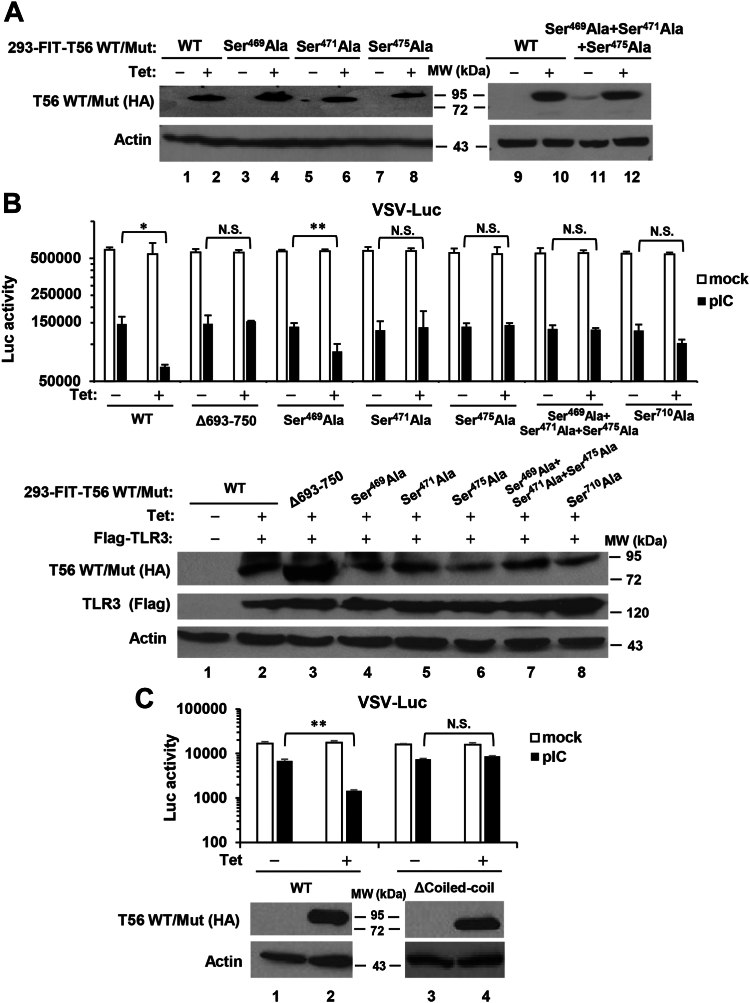
Figure 6.
Impact of alanine substitution of key putative phosphorylation sites or deletion of the Coiled-coil domain on TRIM56-mediated augmentation of TLR3-dependent establishment of an antiviral state.A, characterization of HEK293-FIT-derived cell lines that conditionally express indicated phospho-dead TRIM56 mutant in a Tet-inducible manner. Immunoblot analysis of the expression of WT HA-TRIM56 and various mutant TRIM56 (using mouse anti-HA mAb) in 293-FIT-T56 WT/Mut cells with (+Tet) or without (−Tet) Tet addition for 48 h. Actin serves as a loading control. B, (Upper panel) HEK293-FIT-derived cells conditionally expressing WT HA-TRIM56 and its mutants at the indicated putative phospho-sites with (+Tet) or without (−Tet) Tet, were reconstituted with TLR3 and mock-stimulated or stimulated by poly-I:C, followed by infection with VSV-Luc (MOI = 0.1). At 6 h postinfection, cells were lysed for firefly luciferase assay. (Lower panel) Immunoblot analysis of expression of the induced WT and mutant HA-TRIM56 (using mouse anti-HA mAb) and reconstituted Flag-tagged TLR3 (using mouse anti-flag mAb) in the 293-FIT-T56 WT/Mut cells before VSV-Luc infection. C, HEK293-FIT–derived cells conditionally expressing WT HA-TRIM56 or the Coiled-coil domain deletion mutant (ΔCoiled-coil) cultured with (+Tet) or without (−Tet) Tet were reconstituted with TLR3 and mock-stimulated or stimulated by poly-I:C, followed by infection with VSV-Luc (MOI = 0.1). At 4 h postinfection, cells were lysed for firefly luciferase assay. (Lower panel) Immunoblot analysis of the expression of WT HA-TRIM56 and the ΔCoiled-coil mutant (using mouse anti-HA mAb) in 293-FIT-T56 WT/ΔCoiled-coil cells before VSV-Luc infection. Statistical analysis was performed between poly-I:C-treated −Tet and +Tet cells. Single and double asterisks denote that statistical differences exist with a p value of <0.05 and <0.01, respectively. N.S., not statistically significant; pIC, poly-I:C. TLR, Toll-like receptor; TRIM56, tripartite-motif protein-56; VSV, vesicular stomatitis virus.