Abstract
Background: Acute myeloid leukemia (AML) is a type of blood cancer characterized by excessive growth of immature myeloid cells. Unfortunately, the prognosis of pediatric AML remains unfavorable. It is imperative to further our understanding of the mechanisms underlying leukemogenesis and explore innovative therapeutic approaches to enhance overall disease outcomes for patients with this condition. Methods: Quantitative reverse-transcription PCR was used to quantify the expression levels of microRNA (miR)-133a and miR-135a in 68 samples from 59 pediatric patients with AML. Dual-luciferase reporter transfection assay, Cell Counting Kit-8 assay, and western blot analysis were used to investigate the functions of miR-133a and miR-135a. Results: Our study found that all-trans-retinoic acid (ATRA) promoted the expression of miR-133a and miR-135a in AML cells, inhibited caudal type homeobox 2 (CDX2) expression, and subsequently inhibited the proliferation of AML cells. Additionally, miR-133a and miR-135a were highly expressed in patients with complete remission and those with better survival. Conclusions: miR-133a and miR-135a may play an antioncogenic role in pediatric AML through the ATRA-miRNA133a/135a-CDX2 pathway. They hold promise as potentially favorable prognostic indicators and novel therapeutic targets for pediatric AML.
Keywords: miR-133a, miR-135a, CDX2, acute myeloid leukemia, pediatric, prognosis
Introduction
Acute myeloid leukemia (AML) is a blood cancer characterized by excessive growth of immature myeloid cells and makes up 15% to 20% of childhood acute leukemia. 1 Despite intensified chemotherapeutic regimens and enhanced supportive care measures, and even though allogeneic stem cell transplantation is considered effective for pediatric AML, the overall survival rate for children is still below 70%. 2 In addition, after initial remission, the recurrence rate remains at 15% to 20%. 3 Prognostic biomarkers have the potential to provide valuable insights into the possible outcomes of leukemia, assisting in patient stratification, targeted therapy, and disease monitoring in clinical practice, improving patient prognoses. 4 There is a strong need to identify biomarkers and develop novel biological therapeutic targets to increase pediatric AML survival rates and decrease treatment cytotoxicity.
MicroRNAs (miRNAs) are a class of small noncoding RNA molecules that typically range from 19 to 25 nucleotides in length; they regulate gene expression at the posttranscriptional stage, 5 play a vital role in AML tumorigenesis, and influence hematopoietic cell differentiation and proliferation. 6 It is becoming increasingly clear that miRNAs are involved in leukemia diagnosis, therapeutic response, and prognosis, suggesting their key role in the progression of leukemia.7–9 miR-133a is a muscle-specific miRNA; recent studies have shown that it plays an important role in organ fibrosis, inflammatory responses, and cancer development.10–12 Yang et al found that miRNA-133a is an independent prognostic marker in patients with osteosarcoma and is correlated with cumulative survival. 13 Flavia et al found that miR-133a was expressed at low levels in K562 cells, but was undetectable in HL-60 cells. 14 Interestingly, miR-133a-5p silenced TIM-3 expression, a known biomarker of AML leukemic stem cells. 15 miR-135a is associated with various types of cancer, playing a dual role as a tumor suppressor in some cases while exerting oncogenic functions in others. Low miR-135a levels are associated with a higher risk of AML relapse. 16
Our previous study found that induction of chemotherapeutics such as all-trans-retinoic acid (ATRA) could decrease the activity of homeobox transcription factor caudal type homeobox 2 (CDX2), leading to reduced transcription of miR-125b and enhanced expression of core binding factor β (CBFβ); this promotes myeloid cell differentiation, counteracting leukemogenesis. 17 However, the mechanisms by which ATRA regulates the abnormal expression of CDX2 remain unclear. Our previous miRNA profiling analysis showed that miR-133a and miR-135a were downregulated at diagnosis in pediatric patients with AML compared to healthy controls. 18 Previous studies have also found that ATRA promotes the expression of miR-133a and miR-135a.19,20 These findings raises the possibility that miR-133a and miR-135a might play an intermediary role between ATRA and CDX2 with involvement in the development or pathogenesis of pediatric AML.
In this study, we examined the roles of miR-133a and miR-135a in the ATRA/CDX2/miR-125b/CBFβ pathway and investigated the relationship between miR-133a/135a and complete survival in pediatric patients with AML.
Materials and Methods
Patients and Samples
This study enrolled 59 pediatric patients, including five AML subtypes (AML-M1 to AML-M5), along with 9 healthy controls. Written informed consent was obtained from the parents or guardians of all participants. Diagnoses were established based on clinical features and morphological criteria following the French (American) classification. The biological and clinical characteristics of the patients are presented in Table 1. Bone marrow samples were obtained between January 2007 and April 2014 at the First Affiliated Hospital of Sun Yat-Sen University. This study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (No. [2030] 070). This study conformed to the REMARK guidelines. 21
Table 1.
Characteristics of Patients with AML.
| Stage of the disease | Characteristics | Median (range) | No. (%) |
|---|---|---|---|
| Before therapy | Age at diagnosis, yr | 6.3 (0.3-10) | 38 |
| Sex | |||
| Male | 21 (55.0) | ||
| Female | 17 (45.0) | ||
| WBC count × 109/L | 35.8 (2.8-102.3) | ||
| Less than 10 | 11 (29.0) | ||
| 10-50 | 20 (52.5) | ||
| 50 or higher | 7 (18.5) | ||
| FAB | |||
| M1 | 6 (15.9) | ||
| M2 | 10 (26.2) | ||
| M3 | 15 (39.5) | ||
| M4 | 3 (7.9) | ||
| M5 | 4 (10.5) | ||
| AML1/ETO (M2) | |||
| Positive | 24 (58.8) | ||
| Negative | 10 (17.7) | ||
| Not examined | 4 (23.5) | ||
| Complete remissiona | 13 | ||
| Relapse | 8 | ||
AML: acute myeloid leukemia.
Note. aWith at least 3 years of clinical follow-up.
Cell Culture and RNA/Protein Isolation
NB4 and HL-60 (human myeloid leukemia cell lines) were cultured in RPMI-1640 (HyClone, UT, USA). Both cultures were supplemented with 10% fetal bovine serum (HyClone) and incubated in a 5% CO2 atmosphere at 37 °C. Cell lines were treated with 1 µM ATRA at a density of 1 × 106 cells per well. Total RNA and proteins were extracted using Tirol (Invitrogen) according to the manufacturer's instructions.
Quantitative Real-Time PCR Analysis
Quantitative reverse transcription PCR (qRT-PCR) was performed to detect mature miRNAs and CDX2 mRNA. A total of 68 bone marrow samples were used to determine the expression of miR-133a and miR-135a by qRT-PCR, including nine controls and 59 patients with AML (38 at diagnosis, 13 in CR, and 8 in relapse, all of which were not in pairs). Total RNA was quantified using SYBR Premix Ex Taq II (TaKaRa) and the ABI Step One Detection System (Applied Biosystems). Detection of mature miR-133a and miR-135a in total extracted RNA was performed using the Hairpin-itTM miRNA Real-time PCR Quantization Kit (GenePharma, Shanghai, China), which included a stem-loop-like RT primer and miRNA-specific primers. As an internal control, U6 small RNA was amplified using the same method. mRNA quantification was performed using endogenous β-actin mRNA as an internal control within the same system. Each assay was performed in triplicate.
Cell Transfection and Vector Constructs
The following materials were acquired from GenePharma (Shanghai, China): miR-133a, miR-135a mimics, and scrambled oligonucleotides (miR-NC; negative control); H2O (denoted as miR-mock or anti-mock); miR-133a and miR-135a antisense sequence complementary to mature miR-133a and miR-135a (anti-miR-133a/anti-miR-135a); and miR antisense NC (anti-NC; negative control for miR-133a/miR-135a antisense). HEK-293 T cells were cultured in 24-well plates at a density of 1 × 105 cells per well in a complete growth medium (0.5 mL) and transfected with mimics or miR-NC using lipofectamine 2000 (Invitrogen). NB4 cells were cultured using similar methods and electrotransfected with miRNA mimics or antisense miRNAs at a concentration of 100 nM. Transfected cells were harvested at 48 h posttransfection for western blotting. The fragments of the 3`-UTR of CDX2 mRNA containing the wild-type and mutant binding sites for miR-133a and miR-135a were synthesized by Songon (Shanghai, China) and then inserted into the psiCHECK-2 vector (Promega) for functional analysis. Wild-type and mutant insertions were confirmed using DNA sequencing.
Target Prediction and Luciferase Reporter Assay
To identify possible candidate miRNAs targeting CDX2, we employed PICTAR (http://pictar.mdc-berlin.de/) and TargetScan (http://www.targetscan.org/) as prediction algorithms. Transient transfections were performed in HEK-293 T cells, and cotransfection of CDX2 constructs (psi-Check2-CDX2-WT/Mut), psiCheck control, miR-133a or miR-135a, miR-133a, or miR-135a negative control, and the Renilla luciferase plasmid pRL-TK was performed using Lipofectamine 2000 (Invitrogen). A dual-luciferase reporter assay system (Promega) was used to assess luciferase activity. Relative activity was computed by normalizing the firefly luciferase activity as to the Renilla luciferase activity as an internal control.
Western Blot Assay
Cells were collected 48 h after transfections and lysed with protease and phosphatase inhibitors in RIPA buffer (Pierce, Rockford, IL, USA). The same amount of proteins (2 µg) and loading control (Fermentas) were separated by 10% SDS-PAGE and subsequently transferred onto a polyvinylidene fluoride membrane (Milli-pore, Beijing, China). The membranes were incubated with antibodies against CDX2 (1:5000, BioGenex, SanRamon, CA, USA) or β-tubulin (1:1000, Cell Signaling Technology, MA, USA) at 4 ˚C overnight. After 1 h of incubation with an HRP-conjugated secondary antibody (antirabbit, Sigma-Aldrich), an ECL Plus immunoblotting detection system (GE Healthcare Biosciences) was used to detect protein levels.
Cell Proliferation Assays
A Cell Counting Kit-8 (CCK-8) was used to perform a cell proliferation assay (Dojindo Molecular Technologies, Shanghai, China). Cells were transfected by electroporation with 100 nM miR-133a, miR-135a mimics, 200 nM anti-miR-133a, and anti-miR-135a antisense along with the relative doses of miR-NC and anti-NC. After transfection, 1 × 104 cells per well were seeded into 96-well plates and the CCK-8 assay was performed at 24, 48, 72, and 96 h to measure cell proliferation.
Statistical Analysis
Statistical analyses were conducted using SPSS (Version 15.0; Chicago, IL, USA). Luciferase and qRT-PCR data are presented as the mean ± standard deviation (sd) from three independent experiments. Unless otherwise indicated, differences among groups were assessed using Student's t-test. Statistical significance was set at P < .05.
Results
Differing Expression of CDX2 in ALL and AML, Subtypes of AML, and Primary Cells From Patients
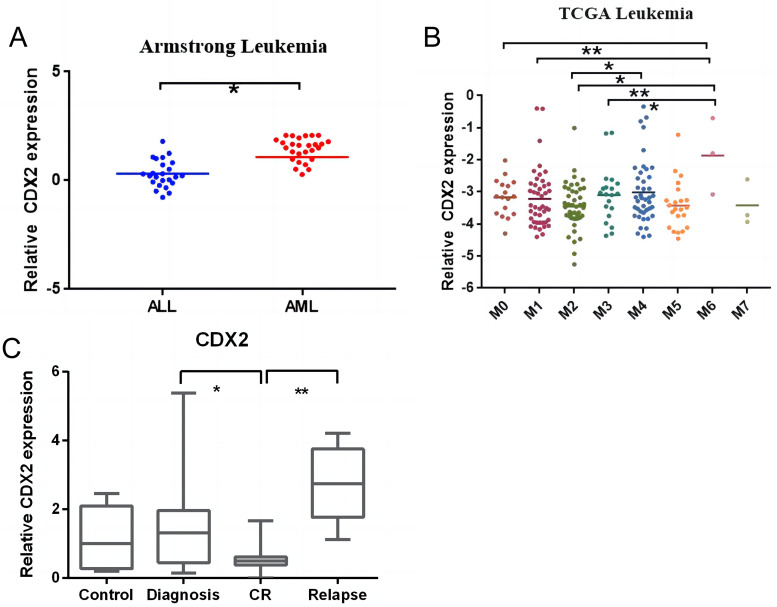
According to the database, CDX2 was more highly expressed in AML than in ALL, particularly in M6 cells (Figure 1A-1B). To confirm that CDX2 acts as an oncogene in AML, we examined the expression of CDX2 in the primary cells of patients. As shown in Figure 1C, CDX2 was expressed at low levels in patients in complete remission compared with samples taken at diagnosis and relapse.
Figure 1.
Differences in expression of CDX2 in ALL and AML, subtypes of AML, and primary cells from patients. (A) Comparison of CDX2 expression between patients with ALL and patients with AML according to the database. (B) CDX2 was highly expressed in AML-M6 compared with other AML subtypes. (C) The expression level of CDX2 in different disease stages in pediatric patients with AML was quantified by qRT-PCR. CDX2 expression was lower in CR patients compared with patients at diagnosis or with relapse. (*P < .05, **P < .01, ***P < .001).
Abbreviation: AML, acute myeloid leukemia.
miR-133a and miR-135a are Predicted to Interact with CDX2
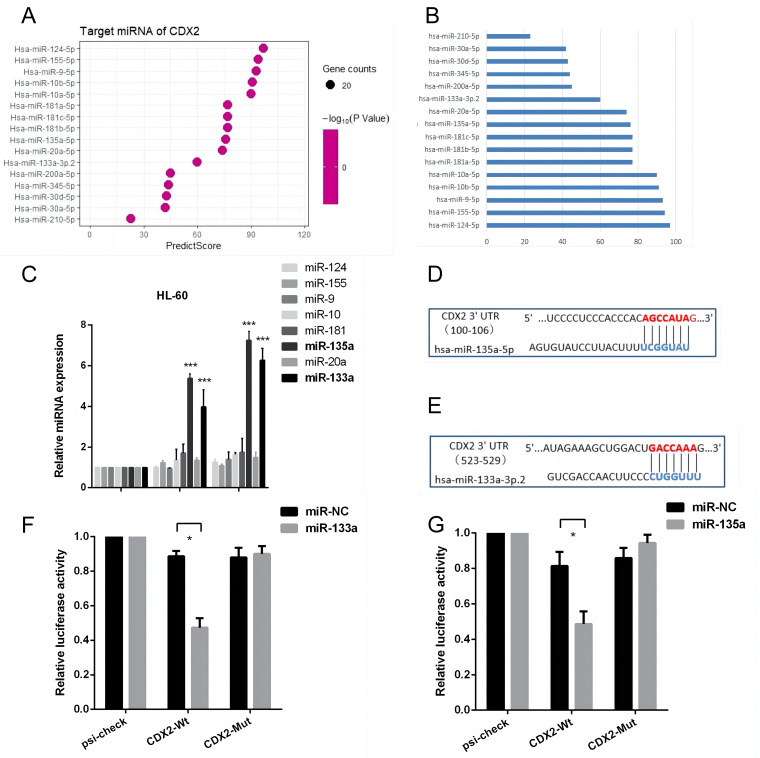
To identify potential molecules targeting CDX2 involved in ATRA-mediated differentiation, we searched miRNA databases for several candidates (Figure 2A-2B). Combining previous research and our experimental results (Figure 2C), miR-133a and miR-135a were computationally predicted to possibly interact with a presumed binding site located in the 3'-UTR of CDX2 (Figure 2D-2E). Luciferase reporter assays were performed to confirm these findings.
Figure 2.
miR-133a and miR-135a are predicted to interact with CDX2. (A/B) TargetScan and PICTAR were used to search for candidate miRNAs. (C) HL-60 cells were treated with ATRA for 48 h, and only miR-133a and miR-135a were significantly expressed. (D/E) miR-133a and miR-135a can target the CDX2 3′UTR. Sequences of the miR-133a and miR-135a binding sites in human CDX2 genomic regions. (F/G) Luciferase reporter assays were employed to confirm the interaction of miR-133a and miR-135a and the CDX2 3′ UTR. Firefly luciferase activity was normalized to Renilla luciferase activity, and the obtained results were presented with reference to the control (NC).
Abbreviation: ATRA, all-trans-retinoic acid.
As shown in Figure 2F-2G, when the wild-type of CDX2 was cotransfected with miR-133a and miR-135a mimics into HEK-293 T cells, both miRNAs significantly repressed luciferase activity compared to miR-NC by 42.7% and 44.1%, respectively. However, with mutant CDX2, the deletion of miR-133a and miR-135a seed recognition sequences did not reduce luciferase activity.
miR-133a and miR-135a Downregulate CDX2 Expression Through Translational Inhibition
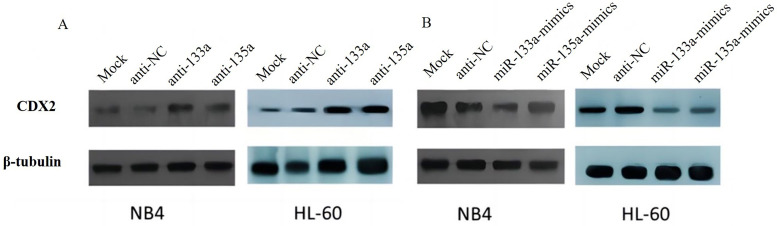
Further verification experiments were performed to determine the potential effects of miR-133a and miR-135a on CDX2 expression. Overexpression and knockdown of miR-133a and miR-135a in NB4 and HL-60 cells, respectively, indicated that the expression of CDX2 protein was significantly reduced in miR-133a- and miR-135a-transfected cells compared with cells transfected with miR-NC and miR-mock (Figure 3B). Transfecting cells with anti-miR-133a and anti-miR-135a upregulated CDX2 protein expression in comparison with anti-NC or anti-mock (Figure 3A).
Figure 3.
miR-133a and miR-135a downregulate CDX2 expression through translational repression. (A/B) The expression level of CDX2 was analyzed by western blotting after the overexpression of miR-133a and miR-135a mimics or the knockdown of endogenous miR-133a and miR-135a using miR-133a and miR-135a antisense in NB4 and HL-60 cells.
miR-133a and miR-135a Inhibit the Proliferation of Two AML Cell Lines
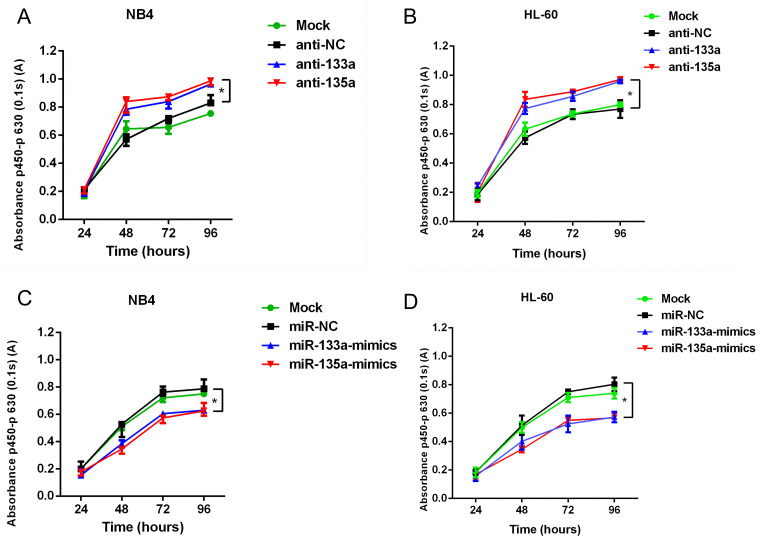
To further demonstrate the potential role of miR-133a and miR-135a in myeloid cell proliferation, we performed a CCK-8 assay on the NB4 and HL-60 cell lines when miR-133a and miR-135a were overexpressed or downregulated, respectively. The results indicated that the survival rates of NB4 and HL-60 cells transfected with anti-133a and anti-135a antibodies were significantly increased (Figure 4A-4B). Cell survival rates in the miR-133a and miR-135a groups were significantly lower than those in the anti-NC and mock groups (Figure 4C-4D).
Figure 4.
miR-133a and miR-135a inhibit the proliferation of two AML cell lines. (A/B) After transfection with anti-133a and anti-135a, the proliferation of NB4 and HL-60 cells was significantly promoted. (C/D) Overexpression of miR-133a and miR-135a inhibited proliferation in NB4 and HL-60 cells.
Abbreviation: AML, acute myeloid leukemia.
miR-133a and miR-135a Are Associated With Different Disease Stages, Subtypes, and Survival
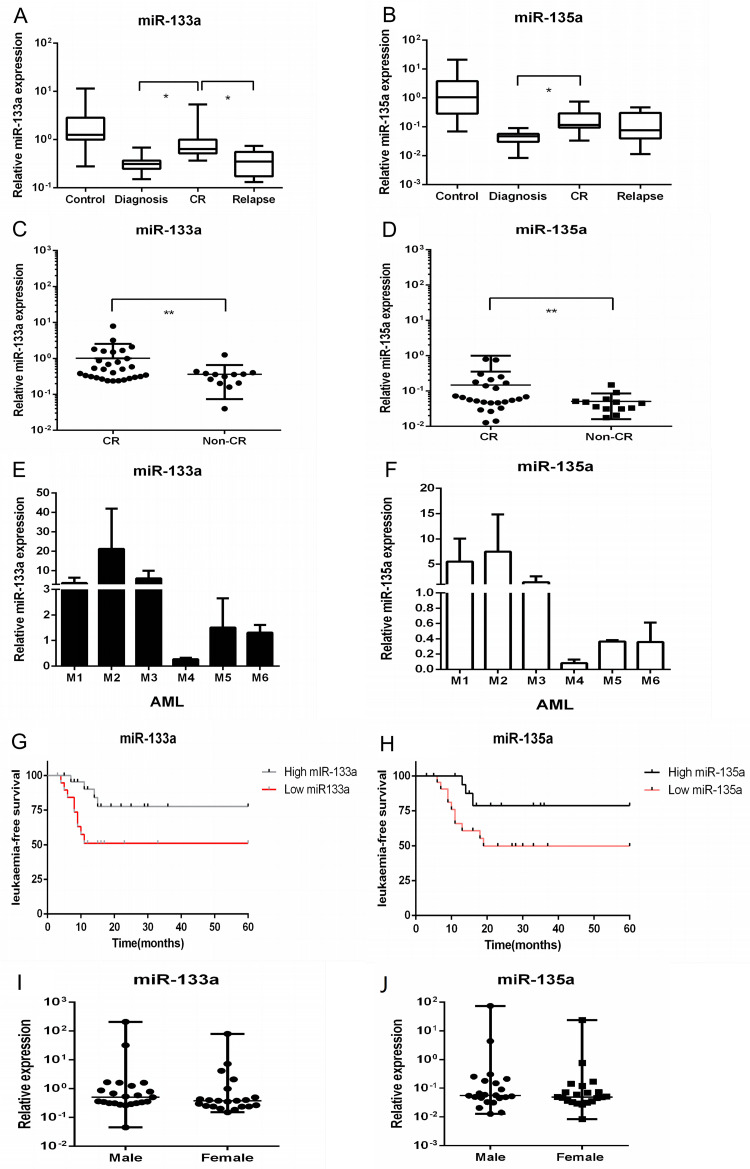
We also investigated the expression of miR-133a and miR-135a at different disease stages and in different subtypes. As shown in Figure 5A-5B, miR-133a and miR-135a were more highly expressed in patients in CR than in patients at diagnosis or with relapse. Figure 5C-5D further demonstrates the significant differences between CR and non-CR. In addition, the two miRNAs had high expression levels in AML subtypes M1-3. Lower expression was observed in other subtypes, including AML M4-5 (Figure 5E-5F).
Figure 5.
miR-133a and miR-135a are correlated with different pediatric AML disease stages, subtypes, and survival. (A/B) The expression of miR-133a and miR-135a was measured by RT-PCR in nine controls and 59 patients with AML. Both were significantly upregulated in CR patients compared to diagnosis and relapse. (C/D) Comparison of the expression of miR-133a and miR-135a between CR and non-CR patients by qRT-PCR. (E/F) MiR-133a and miR-135a were highly expressed in M1-M3 patients in comparison with other subtypes of pediatric AML. (G/H) Patients with elevated expression levels of miR-133a and miR-135a exhibited significantly improved leukemia-free survival (LFS) compared to those with lower expression. (I/J) The expression of miR-133a and miR-135a was not associated with gender.
Abbreviation: AML, acute myeloid leukemia.
A positive correlation was found between elevated expression of miR-133a and miR-135a and improved leukemia-free survival rates during the 5-year follow-up period (Figure 5G-5H). There was no significant difference in expression between male and female mice (Figure 5I-5J).
Discussion
Relapse and failure to respond to first line treatments remain a barrier to survival in children suffering from AML. 22 It is of great importance to clarify the mechanism of leukemogenesis in AML and identify prognostic factors to design novel targeted therapies, predict clinical outcomes, and guide risk-adjusted clinical management.23,24 In recent years, considerable evidence has emerged indicating that miRNAs serve as both oncogenes or tumor suppressors, acting as potential novel biomarkers for leukemia diagnosis, treatment, and prognosis determination.25,26 Our previous studies demonstrated aberrant expression of miR-133a and miR-135a in leukemia cells 18 ; however, few studies have investigated the expression and function of miR-133a and miR-135a in pediatric AML.
The caudal-type homeobox genes are homologous to the caudal-type genes of Drosophila and include CDX1, CDX2, and CDX4. CDX2 is located on chromosome 13q12-13 and consists of three exons and two introns, encoding a protein of 311 amino acids. 27 The CDX2 protein binds to a corresponding DNA region through a helix-loop helix and regulates DNA expression as a transcription factor. 28 CDX2 is primarily recognized as a developmental regulator of early embryogenesis and hematopoiesis.29–31 In normal hematopoietic stem cells (HSC), CDX2 is not expressed; however, it is highly expressed in patients with AML, advanced chronic myeloid leukemia, and high-risk myelodysplastic syndrome.32–34 Aberrant expression of CDX2 has been implicated in promoting the transformation of HSCs into leukemia stem cells, and it is believed that CDX2 might be necessary for leukemia growth. 35 In the present study, we observed aberrant CDX2 expression in pediatric AML, similar to that reported in ectopic expression in adult leukemia. We also identified differential CDX2 expression patterns in different disease stages and subtypes of pediatric AML.
MiR-133a is a confirmed tumor suppressor that has low expression levels in various cancers, such as ovarian, colorectal, bladder, breast, prostate, and esophageal cancers.36–43 miR-135a has also been shown to be a novel tumor regulator; in classical Hodgkin's lymphoma and gastric cancer, miR-135a inhibits tumor cell proliferation by targeting JAK2 to suppress STAT3 activation, decreasing cyclin D1 and Bcl-xL expression.44,45 Additionally, in vitro experiments demonstrated that miR-135a exerted a substantial inhibitory effect on the migratory and invasive capabilities of lung cancer cells. 46 However, in bladder cancer, miR-135a promotes cell proliferation by targeting PHLPP2 and FOXO1, which function as onco-miR. 47 In this study, we found that miR-133a and miR-135a exhibited promising anticancer effects in pediatric AML by directly targeting CDX2, indicating that these miRNAs may be novel therapeutic targets for pediatric AML. We established a correlation between the ATRA-miRNA-CDX2 pathway for the first time, providing fundamental theoretical support for further clarifying the pathogenesis of leukemia and elucidating the mechanism of drug action.
Homeobox (HOX) transcript antisense RNA (HOTAIR) is a lncRNA located on chromosome 12. It can form competitive endogenous RNAs with miRNAs and participate in various biological processes, including proliferation, differentiation, and apoptosis. 48 Liu et al reported that HOTAIR regulates HER2 expression by sponging miR-331-3p in gastric cancer. 49 In addition, Nashwa et al found a significant difference in HOTAIR, c-kit, and miR-193a expression between patients with AML that had 12p abnormalities and those without. Patients with low HOTAIR and c-kit expression exhibited significantly better survival. 50 In contrast, Hu et al found that HOTAIR was significantly downregulated in patients with AML compared to the iron-deficiency anemia control group and that HOTAIR promoted ATRA-induced differentiation by regulating the cell cycle regulator p21 through miR-17-5p. 51 In addition, CDX2 was identified as a transcription factor for HOTAIR in hepatocellular carcinoma by using ChIPBase. 52 Considering this, the potential ATRA/HOTAIR/miRNA/CDX2 pathway deserves further exploration.
We also investigated the relationship between miR-133a and miR-135a expression in different disease stages, subtypes, and survival rates. The results showed that miR-133a and miR-135a were more highly expressed in patients in complete remission than those at diagnosis and with relapse, as well as in patients with better leukemia-free survival. Together, these findings collectively indicate that increased expression levels of miR-133a and miR-135a are associated with a more favorable prognosis and enhanced survival outcomes in pediatric AML. However, this study has some limitations. The mechanism by which ATRA enhances miRNA133a/135a expression remains unclear, and further exploration is required to elucidate the mechanisms underlying leukemogenesis as well as the development of drug resistance and relapse. Additionally, considerable efforts are necessary to discover novel therapeutics, enhance prognosis, and improve overall quality of life.
Conclusion
In conclusion, our study showed that ATRA promoted the expression of miR-133a and miR-135a in AML cells, inhibiting CDX2 expression to inhibit AML cell proliferation. Additionally, high expression of miR-133a and miR-135a were commonly seen in patients in CR and better survival. These findings indicate that miR-133a and miR-135a may serve as potential prognostic markers and novel therapeutic targets in pediatric AML.
Footnotes
Authors’ Note: Yu-Cai Cheng, Zhong Fan, and Cong Liang, contributed equally to this article.
Data Availability: The datasets generated during the study are available from the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Statement: The study was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University. The patients and guardians have signed the informed consent form.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Science and Technology Research Foundation of Guangdong Province, China, the Shenzhen Fundamental Research Program (Grant Nos. A2021328, A2022443, and JCYJ20190809142619278).
ORCID iDs: Zhong Fan https://orcid.org/0000-0003-2632-011X
Chun-Jin Peng https://orcid.org/0009-0002-3722-1133
References
- 1.Reinhardt D, Antoniou E, Waack K. Pediatric acute myeloid leukemia-past, present, and future. J Clin Med. 2022;11(3):16. doi: 10.3390/jcm11030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasche M, Zimmermann M, Borschel L, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32(10):2167-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willier S, Rothämel P, Hastreiter M, et al. CLEC12A and CD33 coexpression as a preferential target for pediatric AML combinatorial immunotherapy. Blood. 2021;137(8):1037-1049. [DOI] [PubMed] [Google Scholar]
- 4.Weng W, Chen Y, Wang Y, et al. A scoring system based on fusion genes to predict treatment outcomes of the non-acute promyelocytic leukemia pediatric acute myeloid leukemia. Front Med (Lausanne). 2023;10:1258038. doi:10.3389/fmed.2023.1258038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Oliveira JC, Roberto GM, Baroni M, et al. MiRNA dysregulation in childhood hematological cancer. Int J Mol Sci. 2018;19(9):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim EL, Trinh DL, Ries RE, et al. Comprehensive sequence analysis of relapse and refractory pediatric acute myeloid leukemia identifies miRNA and mRNA transcripts associated with treatment resistance - a report from the COG/NCI-target AML initiative. Blood. 2015;126(23):3. doi: 10.1182/blood.V126.23.687.687 [DOI] [Google Scholar]
- 7.Cebollada EE, Gómez-González S, Perez-Jaume S, et al. A miRNA signature related to stemness identifies high-risk patients in pediatric acute myeloid leukemia. Br J Haematol. 2023;202(1):96-110. doi:10.1111/bjh.18746 [DOI] [PubMed] [Google Scholar]
- 8.Said F, Tantawy M, Sayed A, et al. Clinical significance of MicroRNA-29a and MicroRNA-100 gene expression in pediatric acute myeloid leukemia. J Pediatr Hematol Oncol. 2022;44(2):E391-E3E5. [DOI] [PubMed] [Google Scholar]
- 9.Zhu RQ, Lin WY, Zhao WW, et al. A 4-microRNA signature for survival prognosis in pediatric and adolescent acute myeloid leukemia. J Cell Biochem. 2019;120(3):3958-3968. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Lu Y, Li Z, et al. MicroRNA-133: expression, function and therapeutic potential in muscle diseases and cancer. Curr Drug Targets. 2014;15(9):817-828. [DOI] [PubMed] [Google Scholar]
- 11.Mitchelson KR, Qin WY. Roles of the canonical myomiRs miR-1, −133 and −206 in cell development and disease. World J Biol Chem. 2015;6(3):162-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Xie W, Wang L, et al. MiRNA-133a aggravates inflammatory responses in sepsis by targeting SIRT1. Int Immunopharmacol. 2020;88:106848. doi:10.1016/j.intimp.2020.106848 [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Qi YB, Si M, et al. A comprehensive analysis for associations between multiple microRNAs and prognosis of osteosarcoma patients. PeerJ. 2020;8:e8389. doi:10.7717/peerj.8389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biamonte F, Zolea F, Santamaria G, et al. Human haematological and epithelial tumor-derived cell lines express distinct patterns of onco-microRNAs. Cellular and Molecular Biology (Noisy-le-Grand, France). 2017;63(11):75-85. [DOI] [PubMed] [Google Scholar]
- 15.Hojati Z, Ganjalikhani-Hakemi M, Ameri M, et al. Evaluation of silencing effect of miR-133a-5p mimic on TIM-3 expression in AML (HL-60) cell line. Indian Journal of Clinical Biochemistry: IJCB. 2020;35(3):359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Beyá M, Brunet S, Nomdedéu J, et al. MicroRNA expression at diagnosis adds relevant prognostic information to molecular categorization in patients with intermediate-risk cytogenetic acute myeloid leukemia. Leukemia. 2014;28(4):804-812. [DOI] [PubMed] [Google Scholar]
- 17.Lin KY, Zhang XJ, Feng DD, et al. miR-125b, a target of CDX2, regulates cell differentiation through repression of the core binding factor in hematopoietic malignancies. J Biol Chem. 2011;286(44):38253-38263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Luo XQ, Zhang P, et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. Plos One. 2009;4(11):e7826. doi: 10.1371/journal.pone.0007826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L-Y, Lin R-Z, Jiang P-F, et al. Identify myeloid differentiation-related MiRNAs response to ATRA induction by RNA sequencing and CRISPR/Cas9 gene editing. Zhongguo shi yan xue ye xue za zhi. 2021;29(2):339-347. [DOI] [PubMed] [Google Scholar]
- 20.Lima L, Tavares De Melo TC, Marques D, et al. Modulation of all-trans retinoic acid-induced MiRNA expression in neoplastic cell lines: a systematic review. BMC Cancer. 2019;19(1):866. doi:10.1186/s12885-019-6081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mcshane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baruchel A, Abrahamsson J, Bertrand Y, et al. Isatuximab in combination with chemotherapy in pediatric patients with relapsed/refractory acute lymphoblastic leukemia or acute myeloid leukemia (ISAKIDS) Interim Analysis. Blood. 2021;138:5. doi:10.1182/blood-2021-150272 [Google Scholar]
- 23.Zarnegar-Lumley S, Caldwell KJ, Rubnitz JE. Relapsed acute myeloid leukemia in children and adolescents: current treatment options and future strategies. Leukemia. 2022;36(8):1951-1960. [DOI] [PubMed] [Google Scholar]
- 24.Egan G, Tasian SK. Relapsed pediatric acute myeloid leukaemia: state-of-the-art in 2023. Haematologica. 2023;108(9):2275-2288. doi:10.3324/haematol.2022.281106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa H, Bhayadia R, Winkler R, et al. Preclinical testing of miRNA-193b-3p mimic in acute myeloid leukemias. Leukemia. 2023;37(7):1583-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esperanza-Cebollada E, Gómez-González S, Perez-Jaume S, et al. A miRNA signature related to stemness identifies high-risk patients in paediatric acute myeloid leukaemia. Br J Haematol. 2023;202(1):96-110. [DOI] [PubMed] [Google Scholar]
- 27.Tomasello G, Barni S, Turati L, et al. Association of CDX2 expression with survival in early colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2018;17(2):97-103. [DOI] [PubMed] [Google Scholar]
- 28.Chawengsaksophak K. Cdx2 animal models reveal developmental origins of cancers. Genes (Basel). 2019;10(11):928. doi: 10.3390/genes10110928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lengerke C, Daley GQ. Caudal genes in blood development and leukemia. Ann N Y Acad Sci. 2012;1266:47-54. doi:10.1111/j.1749-6632.2012.06625.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawengsaksophak K, De Graaff W, Rossant J, et al. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci U S A. 2004;101(20):7641-7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Rowland JE, Van De Ven C, et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell. 2009;17(4):516-526. [DOI] [PubMed] [Google Scholar]
- 32.Rawat VP, Cusan M, Deshpande A, et al. Ectopic expression of the homeobox gene Cdx2 is the transforming event in a mouse model of t(12;13)(p13;q12) acute myeloid leukemia. Proc Natl Acad Sci U S A. 2004;101(3):817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rawat VP, Thoene S, Naidu VM, et al. Overexpression of CDX2 perturbs HOX gene expression in murine progenitors depending on its N-terminal domain and is closely correlated with deregulated HOX gene expression in human acute myeloid leukemia. Blood. 2008;111(1):309-319. [DOI] [PubMed] [Google Scholar]
- 34.Scholl C, Bansal D, Döhner K, et al. The homeobox gene CDX2 is aberrantly expressed in most cases of acute myeloid leukemia and promotes leukemogenesis. J Clin Invest. 2007;117(4):1037-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vu T, Straube J, Porter AH, et al. Hematopoietic stem and progenitor cell-restricted Cdx2 expression induces transformation to myelodysplasia and acute leukemia. Nat Commun. 2020;11(1):3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y, Yao YF, Hu SN, et al. MiR-133a is functionally involved in doxorubicin-resistance in breast cancer cells MCF-7 via its regulation of the expression of uncoupling protein 2. Plos One. 2015;10(6):e0129843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen J, Hu Q, Schrauder M, et al. Circulating miR-148b and miR-133a as biomarkers for breast cancer detection. Oncotarget. 2014;5(14):5284-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, An H, Wang B, et al. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. European Journal of Cancer (Oxford, England: 1990). 2013;49(18):3924-3935. [DOI] [PubMed] [Google Scholar]
- 39.Chiyomaru T, Enokida H, Tatarano S, et al. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102(5):883-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WS, Leung CM, Pan HW, et al. Silencing of miR-1-1 and miR-133a-2 cluster expression by DNA hypermethylation in colorectal cancer. Oncol Rep. 2012;28(3):1069-1076. [DOI] [PubMed] [Google Scholar]
- 41.Wan TM, Lam CS, Ng L, et al. The clinicopathological significance of miR-133a in colorectal cancer. Dis Markers. 2014;2014:919283. doi: 10.1155/2014/919283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshino H, Chiyomaru T, Enokida H, et al. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br J Cancer. 2011;104(5):808-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kojima S, Chiyomaru T, Kawakami K, et al. Tumour suppressors miR-1 and miR-133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer. 2012;106(2):405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Huang M, Cao P, et al. MiR-135a targets JAK2 and inhibits gastric cancer cell proliferation. Cancer Biol Ther. 2012;13(5):281-288. [DOI] [PubMed] [Google Scholar]
- 45.Navarro A, Diaz T, Martinez A, et al. Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood. 2009;114(14):2945-2951. [DOI] [PubMed] [Google Scholar]
- 46.Shi H, Ji Y, Zhang D, et al. MiR-135a inhibits migration and invasion and regulates EMT-related marker genes by targeting KLF8 in lung cancer cells. Biochem Biophys Res Commun. 2015;465(1):125-130. [DOI] [PubMed] [Google Scholar]
- 47.Mao XP, Zhang LS, Huang B, et al. Mir-135a enhances cellular proliferation through post-transcriptionally regulating PHLPP2 and FOXO1 in human bladder cancer. J Transl Med. 2015;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma MZ, Li CX, Zhang Y, et al. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu XH, Sun M, Nie FQ, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Khazragy N, Ghozy S, Matbouly S, et al. Interaction between 12p chromosomal abnormalities and Lnc-HOTAIR mediated pathway in acute myeloid leukemia. J Cell Biochem. 2019;120(9):15288-15296. [DOI] [PubMed] [Google Scholar]
- 51.Hu L, Liu J, Meng Y, et al. Long non-coding RNA HOTAIR regulates myeloid differentiation through the upregulation of p21 via miR-17-5p in acute myeloid leukaemia. RNA Biol. 2021;18(10):1434-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao MR, Han ZP, Liu JM, et al. Bioinformatic analysis and prediction of the function and regulatory network of long non-coding RNAs in hepatocellular carcinoma. Oncol Lett. 2018;15(5):7783-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]