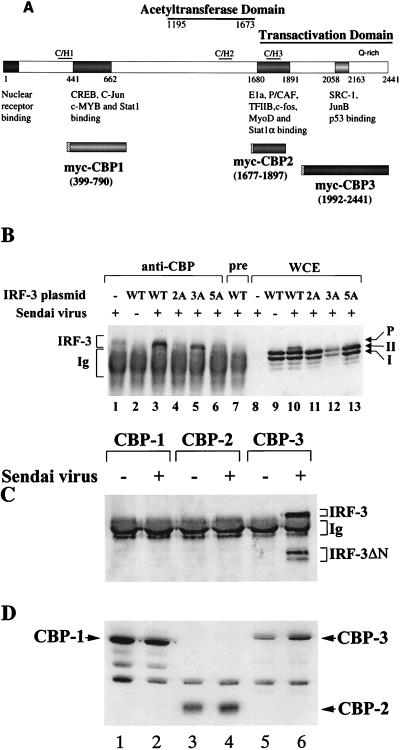
FIG. 8.
IRF-3 interacts with CBP in virus-infected cells. (A) Schematic representation of CBP, illustrating the domains involved in interaction with host or viral proteins (modified from reference 29) and the myc-tagged CBPs (CBP1, CBP2, and CBP3) used for immunoprecipitation. (B) 293 cells were transfected with wild type (WT) and point mutants of IRF-3 expression plasmid (5 μg, as indicated above the lanes) or were left untransfected (lanes 1 and 8). At 24 h after transfection, the cells were infected with Sendai virus for 16 h (lanes 1, 3 to 8, and 10 to 13) or were left uninfected (lanes 1 and 9). Whole-cell extracts (300 μg, except lane 1, which was 600 μg) were immunoprecipitated with anti-CBP antibody A22 (lanes 1 to 6) or with preimmune serum (lane 7). The immunoprecipitated complexes (lanes 1 to 7) or 30 μg of whole-cell extracts (lanes 8 to 13) was run on SDS–5% PAGE gels and subsequently probed with anti-IRF-3 antibody. (C) 293 cells were cotransfected with myc-tagged CBP expression plasmids (as indicated above the lanes) and the IRF-3 ΔN (Δ9-133) expression plasmid. At 24 h after transfection, the cells were infected with Sendai virus (lanes 2, 4, and 6) or were left uninfected (lanes 1, 3, and 5). Whole-cell extracts (300 μg) were immunoprecipitated with monoclonal anti-myc-tag antibody 9E10. The immunoprecipitated complexes were run on SDS–5% PAGE gels, and different forms of IRF-3 in the precipitates were analyzed by immunoblotting with anti-IRF-3 antibody. (D) Whole-cell extracts (30 μg) from panel C were also analyzed directly for the expression of myc-tagged CBP by immunoblotting using anti-myc antibody 9E10.