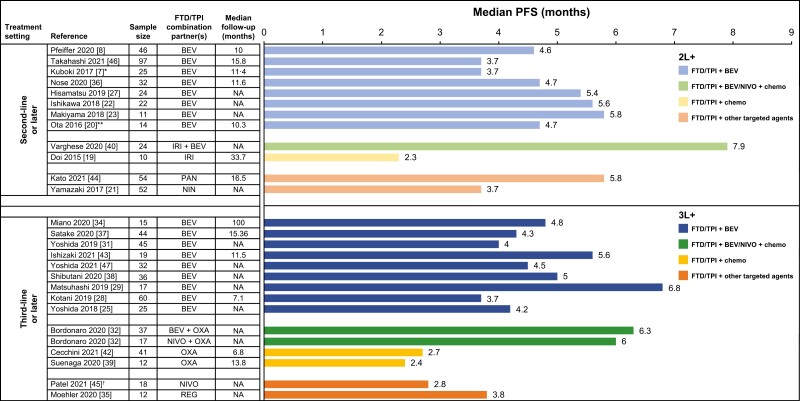
Figure 3.
Median PFS with FTD/TPI-containing combination regimens in patients with metastatic colorectal cancer in second- or third-line settings. *Median PFS was 3.7 by central assessment and 5.6 months by investigator assessment. **Data pertain to patients receiving FTD/TPI ± BEV. †Median PFS was 2.2 months per immune-related response criteria and 2.8 months per response evaluation criteria in solid tumors (RECIST). Abbreviations: 2L, second line; 3L, third line; BEV, bevacizumab; chemo, chemotherapy; FTD/TPI, trifluridine/tipiracil; IRI, irinotecan; NA, not applicable; NIN, nintedanib; NIVO, nivolumab; OXA, oxaliplatin; PAN, panitumumab; PFS, progression-free survival; REG, regorafenib.