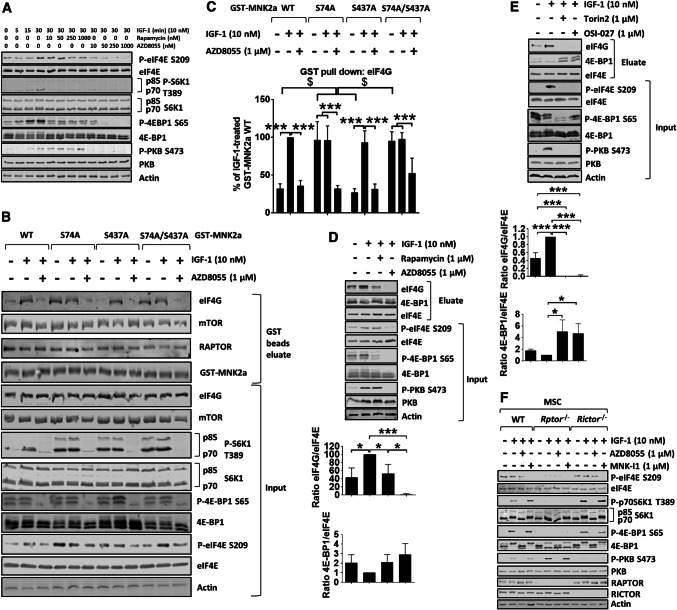
Fig. 3.
mTOR kinase inhibitors, but not rapamycin, reduce eIF4E phosphorylation in vitro and in vivo. a HEK293 cells were starved of serum for 16 h, and then kept in KRB in the presence of the indicated concentrations of rapamycin or AZD8055 for 30 min, before stimulation with IGF-1 (10 nM) for the indicated times. b HEK293 cells were transfected with GST-MNK2a constructs. 32 h later, cells were starved of serum for 16 h and then kept in KRB in the presence of AZD8055 for 30 min, before stimulation with IGF-1 for another 30 min and lysis. GST-MNK2a proteins were then pulled down with glutathione beads. Both eluates and input lysates were subjected to immunoblotting analysis for the indicated proteins. c Quantification of eIF4G binding from b. Results are given as means ± SD. d HEK293 cells were starved of serum for 16 h and then kept in KRB in the absence or presence of rapamycin or AZD8055 for 30 min. eIF4E and associated proteins were then isolated by affinity chromatography on immobilised m7GTP, followed by analysis using SDS-PAGE/WB. e HEK293 cells were starved of serum for 16 h and then kept in KRB in the absence or presence of Torin 2 or OSI-027 for 30 min, followed by stimulation with IGF-1 for another 30 min. eIF4E and associated proteins were isolated by affinity chromatography on immobilised m7GTP, followed by SDS-PAGE/WB. f WT, Rptor−/− and Rictor−/− MSCs were serum starved for 4 h before incubation in KRB in the presence of AZD8055 or MNK-I1 for 30 min, followed by the addition of IGF-1 for another 30 min. Quantification of data in panels c–e is presented as means ± SD, n = 3. *0.01 ≤ P < 0.05; **0.001 ≤ P < 0.01; ***P < 0.001 (one-way ANOVA); $P < 0.001 (two-way ANOVA)