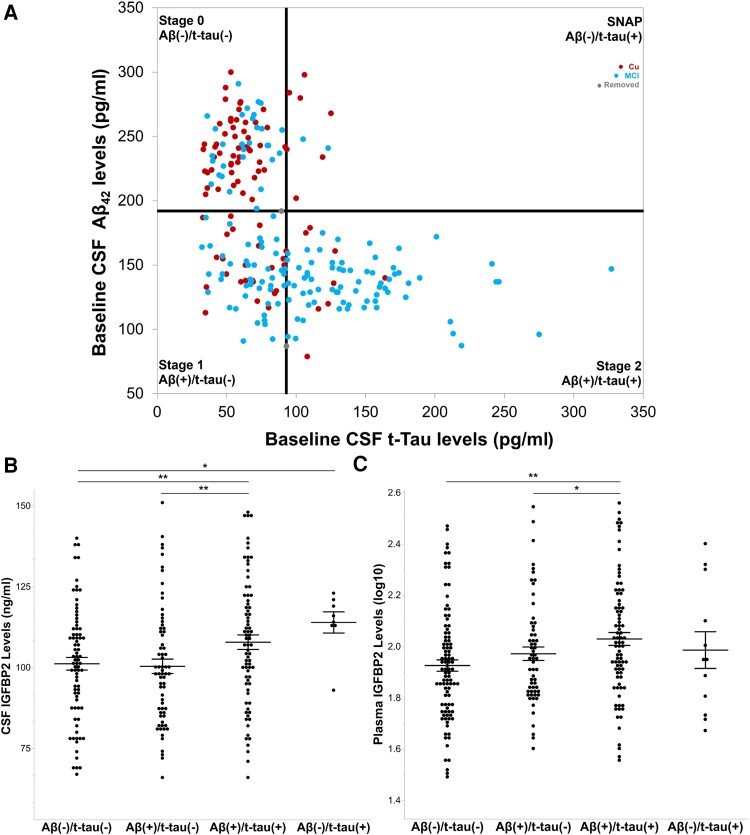
Figure 5.
CSF and plasma IGFBP2 is elevated in CSF Aβ(+)/t-tau(+) individuals from the ADNI-1 cohort. (A) Cognitively unaffected participants (n = 92) and participants with mild cognitive impairment (MCI; n = 149) from the ADNI-1 cohort were staged as CSF amyloid-β and/or CSF total tau-positive according to the recommended thresholds of 192 pg/ml and 93 pg/ml, respectively. Linear models, adjusted for age, sex and APOE ɛ4 carrier status were used to examine mean differences in IGFBP2 protein levels across stages. (B) CSF IGFBP2 was elevated at Stage 2 (n = 77) relative to Stage 0 (n = 80) and Stage 1 (n = 68). Furthermore, CSF IGFBP2 was elevated in suspected non-Alzheimer pathology (SNAP, n = 8) compared with Stage 0. (C) Plasma IGFBP2 was elevated at Stage 2 (n = 84) relative to Stage 0 (n = 98) and Stage 1 (n = 60). However, plasma IGFBP2 did not significantly differ between SNAP (n = 12) and Stage 0. The data are represented as mean ± standard error of the mean. *P < 0.05, **P < 0.01. Aβ42 = amyloid-beta 42; ADNI = Alzheimer's Disease Neuroimaging Initiative; IGFBP2 = insulin-like growth factor binding protein-2; MCI = mild cognitive impairment; SNAP = suspected non-Alzheimer pathology; t-tau = total tau.