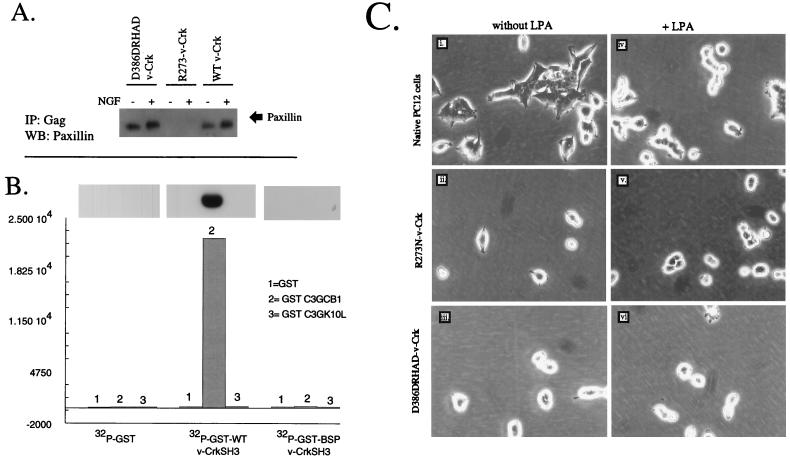
FIG. 3.
Serum- and LPA-induced cell flattening is blocked by mutation in the v-Crk SH2 or SH3 domain. (A) R273N–v-Crk (SH2 mutant) is defective in binding tyrosine-phosphorylated paxillin. D386DRHAD–v-Crk, R273N–v-Crk, or wild-type (WT) v-Crk-expressing cells were kept in the presence or absence of NGF, and the resulting detergent lysates were immunoprecipitated with anti-Gag antibodies and subjected to Western blotting with antipaxillin MAb (arrow). (B) Linker insertion mutations in the Crk SH3 domain disrupt binding to proline-rich peptides derived from the Crk-binding region of C3G. GST or GST fusion proteins containing either wild-type v-Crk SH3 or D386DRHAD SH3 domains were labeled with [32P]orthophosphate (see Materials and Methods). To quantify the binding of the 32P-labeled GST proteins to C3G-derived peptides, 3.5 μg of GST (lanes 1), GST-C3GCB1 (SPPPALPPKKRG) (lanes 2), or GST-C3GK10L (SPPPALPPKLRG) (lanes 3) was electrophoretically resolved in a 13% acrylamide gel and transferred to Immobilon P, and membrane strips were incubated with [32P]GST, [32P]GST–v-Crk SH3, or [32P]GST–D386DRHAD–v-Crk SH3 overnight at 4°C. After being washed, the filters were exposed to X-ray film (autoradiogram) or excised and counted in a β-counter (histograms). (C) Morphological responses of cells expressing v-Crk mutants towards LPA. Native PC12 cells (panels i and iv), R273N–v-Crk cells (panels ii and v), or D386DRHAD–v-Crk cells (panels iii and vi) were grown in serum-free medium for 12 h (panels i to iii) and then treated with 1 μM LPA for 3 h (panels iv to vi). Similar results were obtained with serum (not shown). BSP v-CrkSH3 is th esame as D386DRHAD–v-CrkSH3. The structures and positions of SH2 and SH3 mutations are indicated. Magnification, ×32.