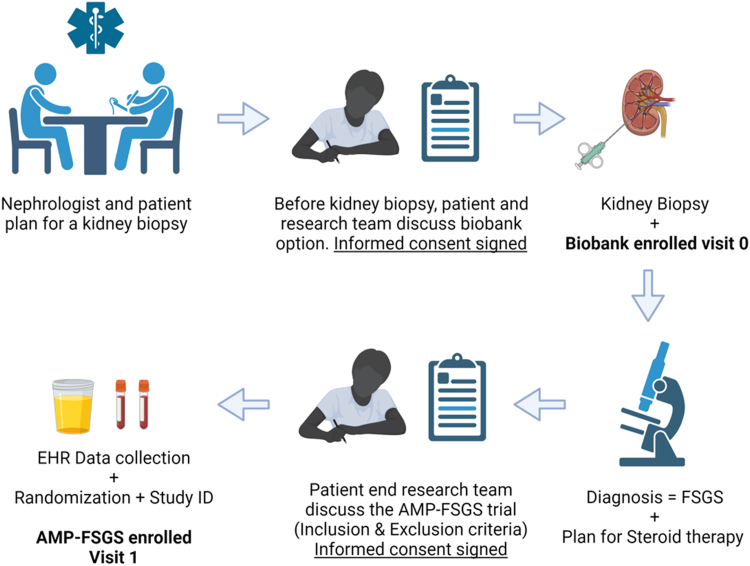
Figure 2.
Enrollment workflow. Schematic describes the enrollment workflow plan from the identification of patients after referral for kidney biopsy by the research team, the approach and consent for biobank enrollment, and sample collection (visit 0). Biobank consent includes optional extra research core for transcriptome evaluation. Once biopsy diagnosis of FSGS (with diffuse FPE) is confirmed and both the treating nephrologist and patient decide on steroid therapy for FSGS (other glomerular diseases are excluded), the research team will approach the patient during clinic visit for enrollment in AMP-FSGS (after evaluating inclusion and exclusion criteria). After AMP-FSGS informed consent is obtained, unique identity generation and randomization will be followed by visit-1 sample collection. FPE, foot process effacement; FSGS, focal segmental glomerulosclerosis.