Fig. 5.
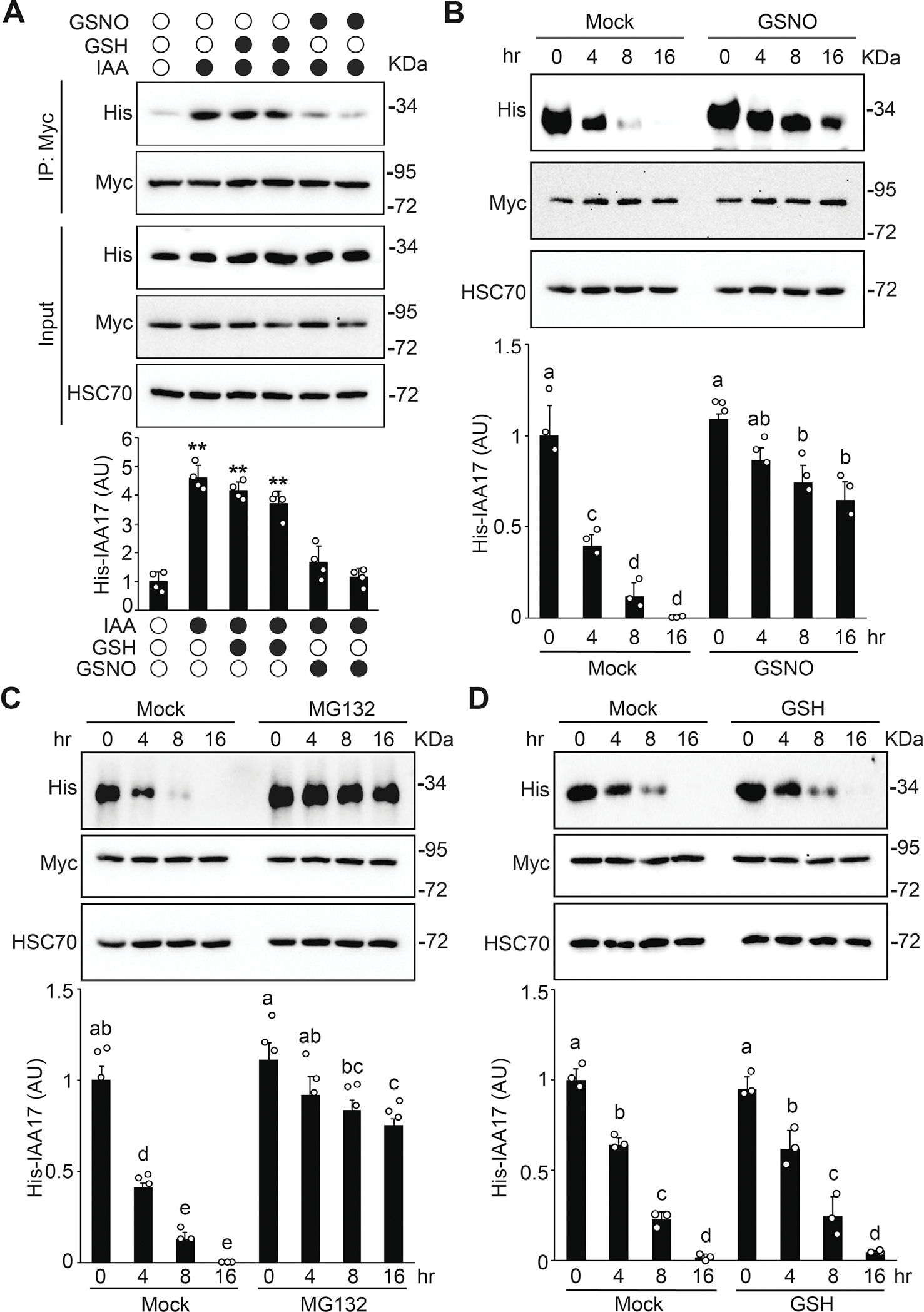
S-nitrosylation negatively regulates IAA17 protein degradation. A: GSNO negatively regulates the auxin-induced TIR1–IAA17 interaction. His-IAA17 recombinant protein was incubated with GSH (negative control) or GSNO for 2 h and then free GSNO was removed by precipitation. TIR1-Myc protein purified from TIR1-Myc transgenic plants by immunoprecipitation was incubated with His-IAA17 recombinant proteins treated with the indicated chemicals. The immunoprecipitated samples were analyzed by immunoblotting using antibodies as indicated. Quantitative analysis of the relative level of His-IAA17 is presented below the blots. Data are mean ± SD of three independent experiments. The statistical significance is determined by a two-sided Student’s t-test (Paired two sample for means). **, P < 0.01 when compared to the mock. B and C: In vitro degradation assay of His-IAA17 recombinant protein. His-IAA17 recombinant protein was incubated with GSNO for 2 h and free GSNO was removed by precipitation. Total protein extracts prepared from TIR1-Myc transgenic plants were incubated with GSNO-treated His-IAA17 recombinant protein for the indicated times in the absence (B) or the presence (C) of 50 μM MG132 for the indicated times. Immunoblotting analysis (top) and quantification (bottom) of His-IAA17 are shown. Anti-HSC70 is used as a loading control. D: In vitro His-IAA17 recombinant protein degradation treated with the negative control GSH as described in (B). Data are mean ± SD of three independent experiments. Different letters indicate individual groups for multiple comparisons with significant differences (one-way ANOVA, Duncan, P < 0.05).
