Figure 2.
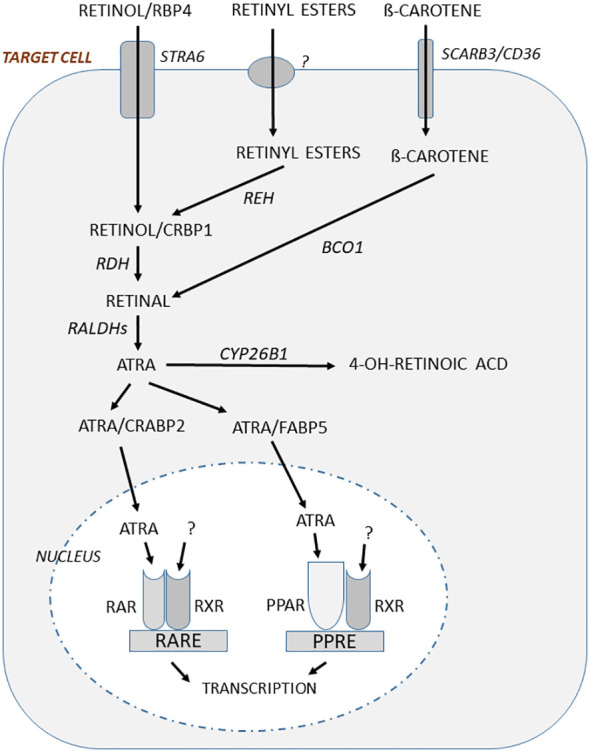
In target cells responding to regulation by vitamin A, retinol bound to RBP4 is taken up through the receptor STRA6 and then bound to CRBP1, whereas ß-carotene uptake is mediated by SCARB3/CD36. Retinyl esters are also taken up, but the mechanism is not known. The retinyl esters are hydrolyzed to retinol by retinyl ester hydrolase (REH). Retinol is oxidized by retinol dehydrogenase (RDH) to retinal which then is oxidized in a two-step processes using retinal aldehyde dehydrogenases (RALDH1, RALDH2) to form ATRA. Retinal is also formed by conversion of ß-carotene through ß-carotene-15,15´-monooxygenase (BCO1). Binding of ATRA to cellular retinoid acid-binding protein 2 (CRABP2) facilitates translocation to nucleus where ATRA binds to RARs. This complex heterodimerize with RXRs for which the ligand is not known, but may be 9-cis-13,14-dihydroretinoic acid. This dimeric ligand-inducible transcription factor will increase or decrease gene transcription through retinoic acid responsive elements (RAREs). ATRA can also bind to fatty acid-binding protein 5 (FABP5) and this complex facilitates translocation to the nucleus and binding to peroxisome proliferator-activated receptors (PPAR), which after heterodimerizing with RXRs act as a transcription factor increasing or decreasing gene transcription through peroxisome proliferator response element (PPRE).
