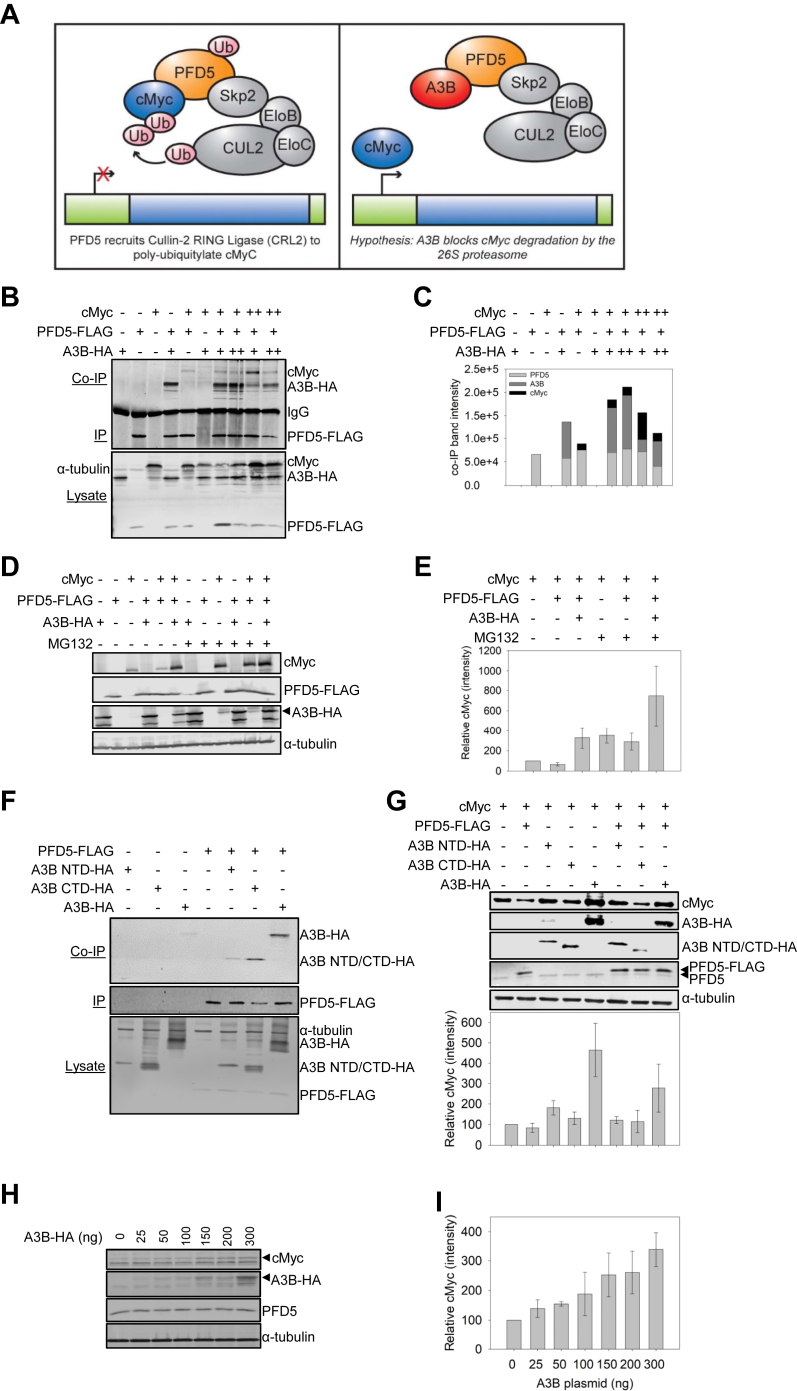
Fig. 4.
A3B inhibits PFD5-mediated degradation of cMyc.A, left: Diagram of tumor suppressor PFD5 recruiting Cullin-2 RING ubiquitin ligase for cMyc ubiquitination and degradation. Right: A3B interacts with PFD5 leading to the hypothesis that A3B can disrupt this degradative pathway and lead to A3B-mediated, but deamination-independent, dysregulation of the cell cycle. B and C, co-IP experiments from HEK293T cells treated with 12.5 μM MG132 for 16 h demonstrated that A3B-HA and cMyc each interact with PFD5-FLAG. Using combinations of transfections of low A3B (1 μg, +), high A3B (2 μg, ++), low cMyc (1 μg, +), or high cMyc (4 μg, ++) demonstrated that the presence of A3B resulted in less co-purification of cMyc with PFD5 in a concentration-dependent manner. C, is the quantification of (B). D and E, this interaction was functional and resulted in more cMyc in cells in the presence of A3B-HA, when PFD5-FLAG was also present. The experiment was performed with or without MG132 treatment (12.5 μM for 16 h) as indicated above the blot (D) or graph (E). E, the quantification of three independent blots, with a representative blot shown in (D). F, co-IP experiments from HEK293T cells demonstrated that A3B NTD-HA and A3B CTD-HA both interact with PFD5-FLAG. G, The A3B NTD-HA and A3B CTD-HA interactions with endogenous PFD5 and PFD5-FLAG were not functional and did not result in more cMyc in cells. Only the presence of full-length A3B-HA could fully recover cMyc levels. G, is the quantification of three independent blots, with a representative blot shown. H and I, A3B-HA regulation of endogenous PFD5 occurs in the MCF7 breast cancer cell line. MCF7 cells were transfected with increasing amounts of A3B-HA. The endogenous cMyc detected increased with the amount of A3B-HA protein in cells. The quantification of two independent blots is plotted in (I). A representative blot is shown in (H). E, G and I, immunoblots were quantified using Image Studio software with normalization of each experimental lane to its respective anti-α-tubulin band. Normalized values were then converted to relative amounts by setting the cMyc alone condition to 100 and calculating the relative cMyc in other experimental conditions.