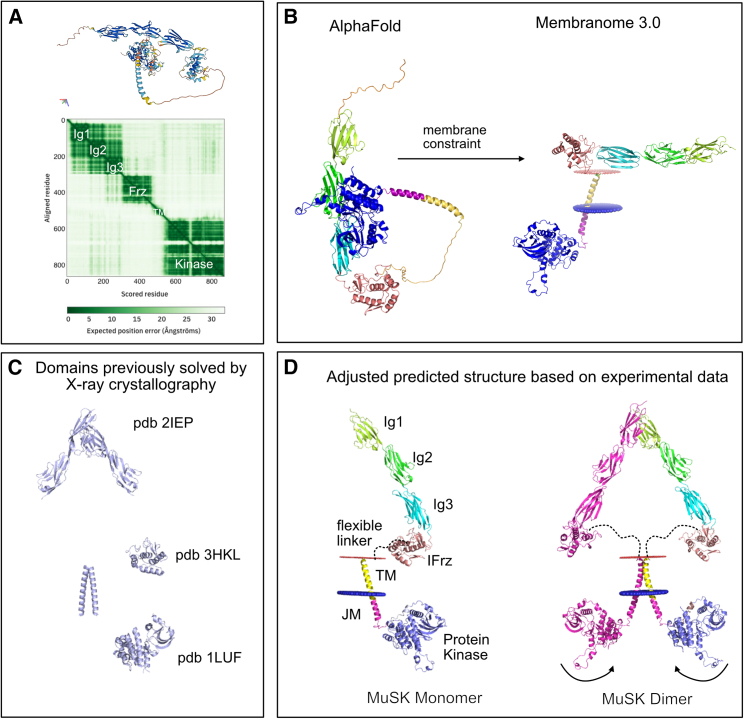
Figure 2.
Modeling the structure of MuSK using experimental and predicted data suggests dimerization occurs at both the Ig1 domain and the transmembrane domain
(A) The MUSK_HUMAN structure from the AlphaFold database (https://alphafold.ebi.ac.uk/entry/O15146) has a biologically unlikely orientation with the intracellular kinase domain interacting closely with the extracellular Ig2 domain.
(B) Membranome removes disordered regions and forces a membrane constraint to correctly separate the intracellular and extracellular domains; however, without the flexible linker, which is present between the extracellular domain and transmembrane domain, the extracellular portion of the protein is positioned horizontally along the membrane, which would not allow for Ig1 dimerization concurrently with kinase trans-autophosphorylation.
(C) Previously solved domains by X-ray crystallography were obtained from the RCSB Protein Data Bank (https://rcsb.org).
(D) Allowing for the flexible linker between the Frz-like and transmembrane domain to modify the position of the extracellular domain allows for an orientation of the MuSK protein that is biologically plausible and allows for dimerization at the Ig1 domain, within the transmembrane domain, and within reach for trans-autophosphorylation of the kinase domains.