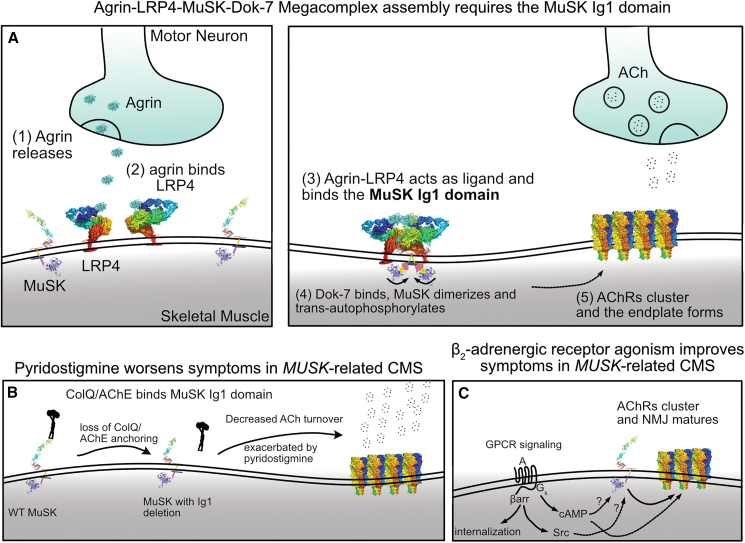
Figure 5.
Conceptual models suggest the importance of the Ig1 domain for MuSK protein function
(A) The formation of the agrin-LRP4-MuSK-Dok-7 megacomplex occurs stepwise. Unlike most receptor tyrosine kinases, which bind directly to a ligand, agrin first binds LRP4, and the agrin-LRP4 complex serves as a ligand for MuSK. Following agrin-LRP4-MuSK binding on the extracellular side, Dok-7 binds intracellularly, leading to MuSK activation by trans-autophosphorylation (i.e., the kinase domains from opposite MuSK monomers phosphorylate each other). Activated MuSK phosphorylates downstream targets to result in AChR clustering and endplate formation.
(B) The Ig1 domain is the site of binding for ColQ, allowing for proper localization of AChE in the synapse. This suggests a model to explain why our patient experienced severe worsening in the setting of a trial of pyridostigmine (an AChE inhibitor).
(C) Systemic β-agonist therapy is the current mainstay of therapy, though the mechanism of signaling through the β-adrenergic receptor is not completely known. Whether G-protein signaling, β-arrestin signaling, or both are involved is an open question.