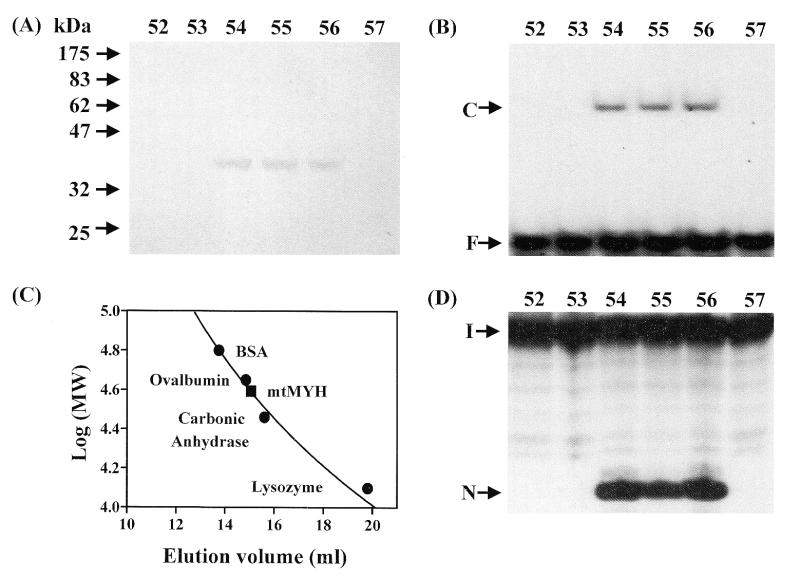
Figure 2.
Purification of calf mtMYH by Superose 12 column. Calf mtMYH (fraction V) was purified as described in Materials and Methods and loaded onto a 24.5 ml calibrated Superose 12 gel filtration column and 275 µl fractions were collected. (A) SDS–polyacrylamide analysis. Superose 12 column fractions were concentrated by TCA precipitation, electrophoresed on a 10% SDS–PAGE and stained with silver. The positions of the molecular weight standards (New England Biolabs prestained markers) are marked. (B) A/8-oxoG binding activity of the fractions from Superose 12 column. The positions of free DNA (F) and binding complex (C) are marked. (C) The native molecular mass of mtMYH was estimated to be 35–40 kDa. The Superose 12 column was calibrated with blue dextran (2000 kDa), BSA (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (29 kDa) and lysozyme (14 kDa). Molecular weight markers (marked by filled circles) were monitored by UV absorption and mtMYH (marked by a filled rectangle) was analyzed by A/8oxo-G binding activity. (D) A/8-oxoG glycosylase activity of the fractions from Superose 12 column. The samples in the loading dye were heated at 90°C for 2 min and electrophoresed in a 14% sequencing gel. The positions of intact DNA substrate (I) and cleaved products (N) are marked. The numbers above (A), (B) and (D) represent the fraction number eluted from the Superose 12 column.