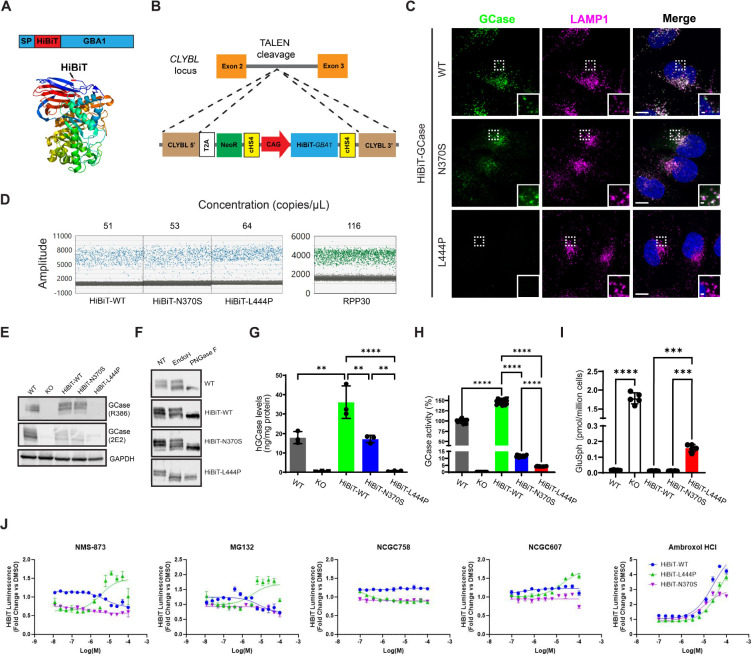
Figure 1. HiBiT-tagged GCase retains normal trafficking and function, enabling a high-throughput screening assay measuring cellular GCase levels.
(A) GCase was labeled with a small (1.3 kDa), pro-luminescent, N-terminal HiBiT peptide tag immediately following the signal peptide (SP) sequence. (B) This HiBiT-GCase reporter (WT, N370S, or L444P) was engineered into a human Citrate Lyase Beta-Like (CLYBL) intragenic safe-harbor locus within a GBA1-KO H4 cell background using TALEN-enhanced integrative gene transfer. (C) Co-localization of GCase (green) with lysosomal marker LAMP1 (magenta) was determined by immunofluorescent staining. (D) The copy number of the stably-integrated transgene was confirmed to be 1 across all three HiBiT-GCase lines via Droplet Digital polymerase chain reaction (ddPCR). The HiBiT-GCase H4 lines featured ~ 60 copies/μL of the HiBiT-GBA1 transgene, as compared with ~ 120 copies/μL of the reference gene RPP30, which has a known copy number of 2 in the GBA1-WT H4 cell line. (E) GCase protein level was measured by Western blot in GBA1-WT, GBA1-KO, and HiBiT-GCase H4 cell lines using anti-GCase (R386, 2E2) antibodies. (F) Glycosidase sensitivity analysis indicates that HiBiT-GCase-L444P is entirely retained in the ER. The Endo H-sensitive fraction (lower band) on the blot contains immature, ER-retained GCase, while the Endo H-resistant fraction (top band) contains maturely-glycosylated, post-ER-localized GCase. Both fractions are responsive to PNGase F treatment. NT: non-treated. (G) GCase protein levels were quantitated by AlphaLISA (Amplified Luminescent Proximity Homogeneous Assay) utilizing a sandwich configuration of two monoclonal antibodies recognizing non-overlapping epitopes, hGCase-1/23 (which was biotinylated and associated with a streptavidin-coated donor bead) and hGCase-1/17 (which was directly conjugated to an acceptor bead). (Error bars: SEM [n = 3 biological replicates]). (H) GCase activity was measured in cell lysates using the fluorogenic substrate 4-methylumbelliferyl-β-D-glucopyranoside. Relative GCase activity was calculated by adjusting for protein concentration, correcting for GBA1-KO H4 cell background, and normalizing to GBA1-WT signal. (Error bars: SD [n = 16 technical replicates]). (I) Levels of glucosylsphingosine (GluSph) in H4 cell pellets were quantified by positive ion electrospray LC-MS/MS in multiple reaction-monitoring mode, using deuterated compounds as internal standards. (Error bars: SD [n = 5 biological replicates]). (J) Pilot testing of the HiBiT-GCase assay was performed in ultra-high-throughput 1536-well plate format. Cells were treated with known-active ERAD modulators (NMS-873, p97 inhibitor; MG132, proteasome inhibitor) or GCase stabilizers (ambroxol, NCGC758, or NCGC607) for 24 h, followed by measurement of HiBiT-GCase luminescence. For each respective cell line, data are represented as fold change in luminescence (RLU) in compound-treated versus DMSO-treated cells. (Error bars: SEM [n = 3 – 6]). Dose-response curves were fit using log(agonist) vs. response (three parameters). *P-value ≤ 0.05; **P-value ≤ 0.01; ***P-value ≤ 0.001; ****P-value ≤ 0.0001.