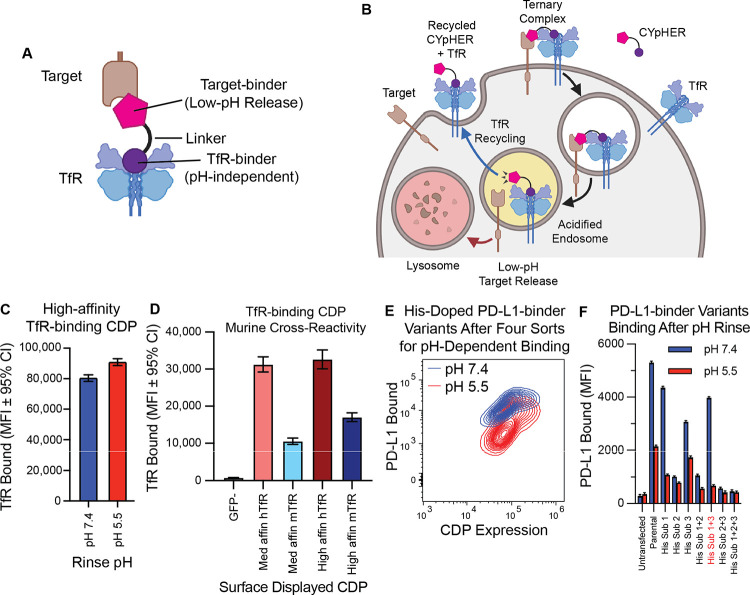
Fig. 1. Basic principles of CYpHER and component binders.
(A) CYpHER design including a pH-independent TfR-binding domain and a pH-dependent target-binding domain separated by a linker. (B) CYpHER mechanism. CYpHER induces ternary complex formation with target and TfR. Upon TfR-mediated uptake and endosomal acidification, target is released for endolysosomal system trafficking. TfR and CYpHER recycle to the surface for engagement with another target molecule. (C) 293F cells displaying a high-affinity TfR-binding CDP were stained with TfR and rinsed at pH 7.4 or pH 5.5 for 10 minutes, showing similar binding in both conditions. (D) 293F cells displaying medium or high affinity TfR-binding CDPs were stained with human TfR (hTfR) or mouse TfR (mTfR). (E) pH-dependent PD-L1 binding flow profile of 293F cells displaying a pool of histidine-doped variants of a PD-L1-binding CDP after four rounds of flow sorting; two for high binding after pH 7.4 rinse, two for low binding after pH 5.5 rinse. (F) Three His substitutions were tested as singletons and combinations for PD-L1-binding after 10 minutes pH 7.4 or pH 5.5 rinse. Variant with His substitutions 1 and 3 was chosen for further work.