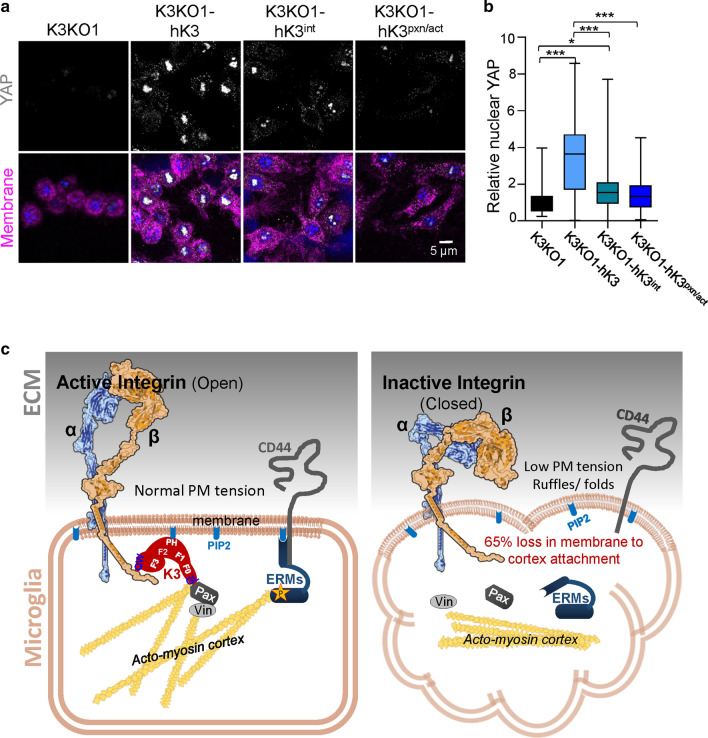
Fig. 7.
K3 regulates YAP localization to the nucleus. a Confocal images of RAW cells (K3KO, K3KO-hK3. K3KO-hK3int and K3KO-hK3pxn/act) spread on fibronectin for 24 h and stained for YAP (gray), nuclei (DAPI, blue), and membrane with WGA-647 (magenta). b Quantification of YAP localized to the nucleus of RAW cells (n = 60 cells from three experiments). c Proposed role for K3 in regulation of MCA: K3 localizes to the membrane by binding to cytoplasmic domain of β-integrin (via QW residues in F3 domain) and PIP2 (via PH domain). Upon binding integrin, K3 recruits actin and paxillin (Pax) to its F0 domain (via the highly conserved GL residues) leading to integrin activation (open conformation). Paxillin, possibly via vinculin (Vin), further reinforces K3′s connection with the acto-myosin cortex. These events lead to the phosphorylation and activation of ERMs via effector kinase. Upon phosphorylation, ERMs undergo a conformational change, bind to actin, and localize to the membrane by binding PIP2 and transmembrane receptors such as CD44. The absence of K3 results in loss of MCA, low apparent membrane tension, ERMs mislocalization and membrane ruffling