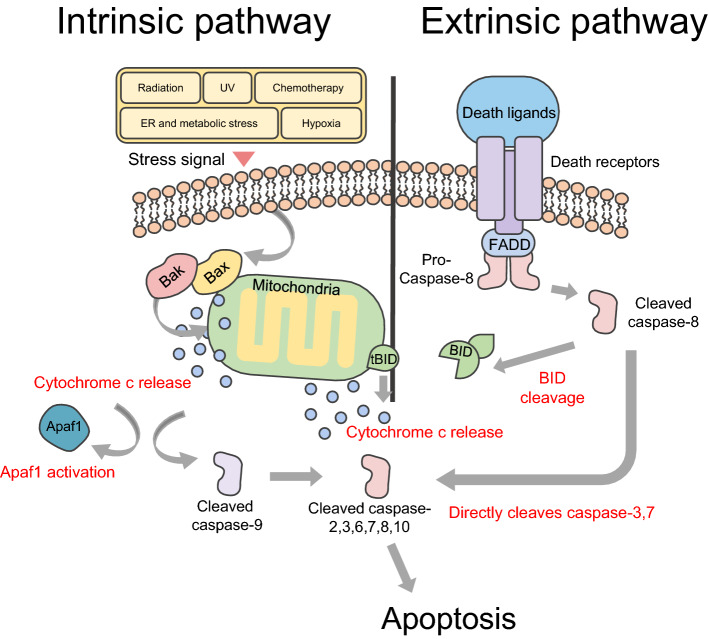
Fig. 1.
Extrinsic and intrinsic pathways in apoptosis. Internal stimuli, including DNA damage, activate apoptotic proteins belonging to the Bcl-2 family Bax and Bak. And this induces formation of Bax-Bak complexes in the mitochondrial outer membrane. The complexes release cytochrome c and enhance levels of apoptotic protease activator 1 (Apaf1). Released cytochrome c promotes proteolytic maturation of the activator caspase-9. Caspase-9 then cleaves and activates other effectors, caspase-2, 3, 6–8, and 10, and consequently induces apoptosis, and this series of processes is called the intrinsic pathway in apoptosis. As the death receptor and ligand bind, FAS-associated death domain protein (FADD) and pro-caspase-8 line up to bind to the intracellular portion of the death receptor. At this time, the recruited caspase 8 can directly cleave and activate caspase-3 and caspase-7 and proteolytically activate the BH3-only protein BH3-interacting domain death agonist (BID). Truncated BID (tBID) promotes mitochondrial membrane permeability through activation of the Bax-Bak complex of the outer mitochondrial membrane, and then induces apoptosis through cleavage and activation of caspases, and this series of processes is called the extrinsic pathway in apoptosis