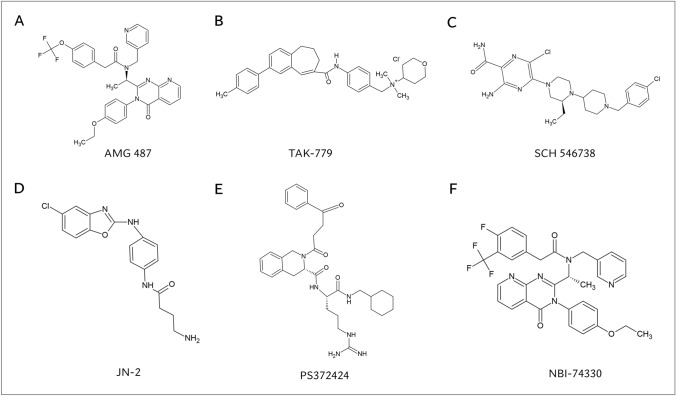
Fig. 4.
Chemical structures of the small-molecule CXCR3 antagonists and CXCR3 agonist that were evaluated in rodent models of arthritis. Chemical structures of A small-molecule CXCR3 antagonist AMG 487, B small-molecule CCR5/CXCR3/CCR2 antagonist TAK-779, C small-molecule CXCR3 antagonist SCH 546,738, D small-molecule CXCR3 antagonist JN-2, E small-molecule CXCR3 agonist PS372424, and F small-molecule CXCR3 antagonist NBI-74330. AMG 487, N-1-[(3-4(-Ethoxyphenyl)-3,4-dihydro-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-N-(3-pyridinylmethyl)-4-(trifluoromethoxy)benzeneacetamide; JN-2, N-(4-(5-chlorobenzo[d]oxazol-2-ylamino)phenyl)-4-aminobutanamide; NBI-74330, N-1-[(3–4(-Ethoxyphenyl)-3,4-dihydro-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-4-fluoro-N-(3-pyridinylmethyl)-3-(trifluoromethyl)benzene-acetamide; PS372424, (S)-N-((S)-1-((cyclohexylmethyl)amino)-5-guanidino-1-oxopentan-2-yl)-2-(4-oxo-4-phenylbutanoyl)-1,2,3,4-tetrahydro-isoquinoline-3-carboxamide; SCH 546738, 3-Amino-6-chloro-5-[(3S)-4-[1-[(4-chlorophenyl)methyl]-4-piperidinyl]-3-ethyl-1-piperazinyl]-2-pyrazinecarboxamide; TAK-779; N, N-dimethyl-N-(4-[[[2-(4-methylphenyl)-6, 7-dihydro-5H-benzocyclohepten-8-yl]carbon-yl]amino]benzyl)-tetrahydro-2H-pyran-4-aminium chloride