Fig. 2.
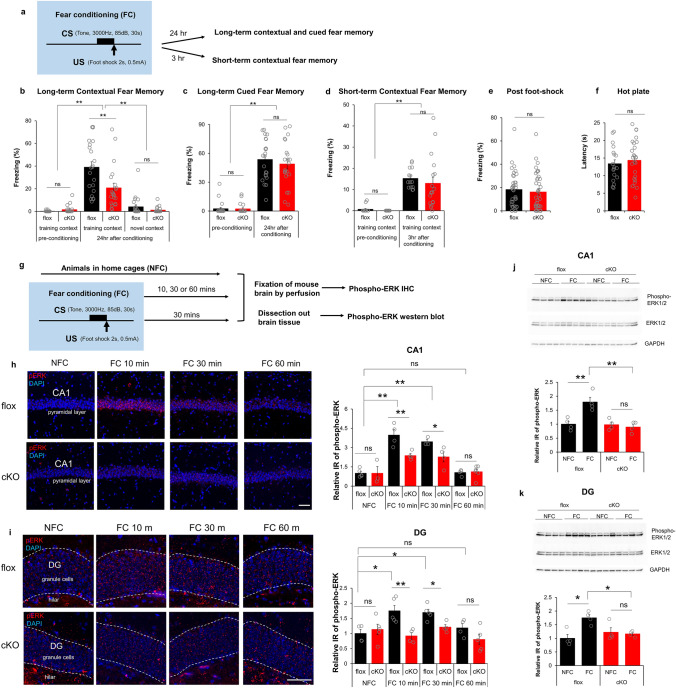
Camk2α-cre+/-::RapGEF2fl/fl (cKO) mice show deficit in consolidation of contextual fear memory. a–f Consolidation of contextual fear memory was impaired in Camk2α-cre+/-::RapGEF2fl/fl mice. The scheme of a fear conditioning test used for cKO and flox control mice (a). cKO mice showed impaired contextual memory to the training context, not novel context, 24 h later compared to controls (b). However, both flox and cKO mice showed normal levels of freezing during the tone presentation in a non-training context when memory was retrieved 24 h after conditioning (c). Two-way ANOVA following by post hoc Bonferroni t-test, **p < 0.001. N = 22 for animal number of flox mice, N = 21 for animal number of cKO mice. Contextual freezing 3 h after the conditioning was similar between cKO and flox mice, suggesting acquisition and retrieval of memory was not affected in cKO mice (d). Two-way ANOVA following by post hoc Bonferroni t-test, **p < 0.001. N = 14 for animal number of flox mice, N = 18 for animal number of cKO mice. Flox and cKO mice showed similar freezing level immediately after foot-shock during fear conditioning (e, N = 36 for flox mouse number, 39 for cKO mouse number) and similar latency in a hot plate test (f, N = 19 ~ 26 for animal number in each group), suggesting no differences in pain sensitivity between two groups. g–k ERK activation in hippocampus during fear conditioning is RapGEF2-dependent. The Experimental procedure to examine ERK activation after fear conditioning (g). Representative images of phospho-ERK staining (in red) in hippocampal CA1 pyramidal cell layer (h, panels on the left) or hippocampal DG granule cell layer (i, panels on the left) of flox and cKO mice 10 min (FC10 min) or 30 min (FC 30 min) or 60 min (FC 60 min) after fear conditioning or without fear conditioning (NFC). Scale bar: 50 µm. Immunoreactive (IR) signals of phospho-ERK in the CA1 or DG of flox and cKO mice at different time points after fear conditioning were quantified by NIH Image J using the mean gray values of integrated density after being converted to gray scale; then compared to average value from mice in the home cage (NFC) to obtain “Relative IR of phospho-ERK” (h and i, panels on the right). N = 3 ~ 5 for animal number in each group. Two-way ANOVA following by post hoc Bonferroni t-test, *p < 0.05; **p < 0.001. Phospho-ERK activation in hippocampal CA1 and DG were also quantified with western blot (j and k). Hippocampal CA1 or DG were dissected out from flox or cKO mice 30 min post fear conditioning (FC) or without fear conditioning (NFC). Protein lysates were subjected to western blots with phospho-ERK, pan-ERK and GAPDH antibodies. N = 4 for animal number in each group. Protein bands from western blots were quantified using imageJ and GAPDH protein served as internal controls to normalize the loading. Phospho-ERK IR from different groups was compared to average value from flox mice in the home cage (NFC), to obtain “Relative IR of phospho-ERK”. Two-way ANOVA following by post hoc Bonferroni t-test, *p < 0.05; **p < 0.001