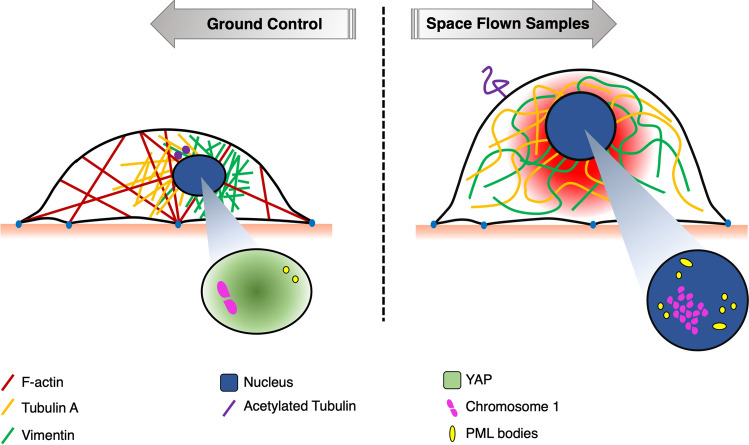
Fig. 9.
A model of subcellular events in space flight. Location of subcellular components at any time depends on the integrity and integration of structural contribution of cytoskeletal compartments (microtubules, intermediate filaments, microfilaments). The position of the nucleus depends on the intermediate filaments network, which together with other scaffold proteins, modifies gene transcription. At 1g, compressive or stretching forces are mechanically balanced by cytoskeletal reorganization to provide the internal resistance to nucleus sedimentation. In space, the response caused by unloading is transmitted likewise to/from the nucleus through the cytoskeleton. If we represent the cell as a nucleated tensegrity structure, we expect exposure to microgravity to cause sudden nuclear repositioning. Later on, chronic exposure elicits adaptive changes, likely initiated by cytoskeleton-bound signaling. In summary, this study reports a general softening of a model of microvascular ECs in SF, with reduction of cell motility. The general reorganization of the cytoskeleton showed remarkable loss of actin stress fibers and compensative enlargement of microtubules and intermediate filaments. Cell signaling was altered, at least concerning Hh, with increased frequency of primary cilia. DNA damage repair mechanisms were activated (PML bodies) and even saturated (γH2AX). Territories of marker chromosomes were found altered compared to GCs