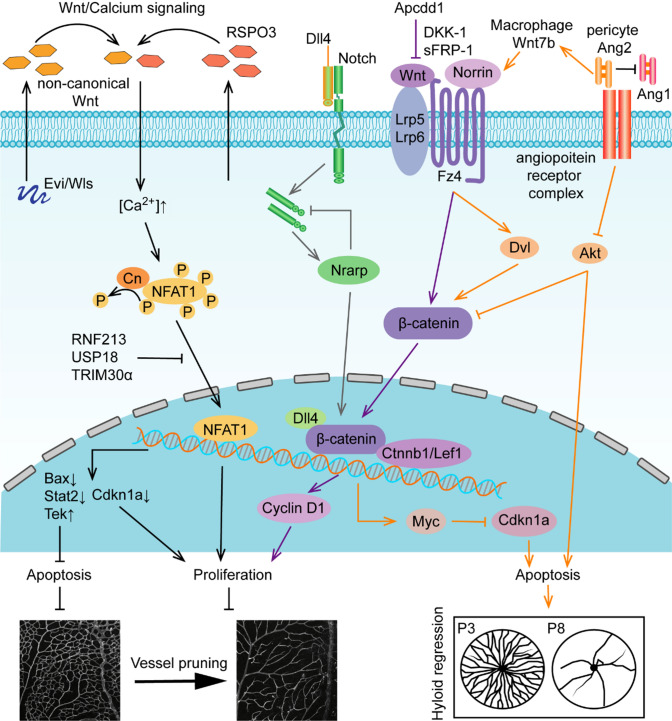
Fig. 6.
Role of Wnt signaling in vessel remodeling. Non-canonical Wnt ligands activate Wnt/Ca2+ signaling and regulate vessel remodeling at the transcriptional level of apoptosis- and proliferation-related genes. Evi/Wls/R-spondin3 (Rspo3) activate non-canonical WNT/calcium signaling at the level of NFAT1 by downregulating Rnf213, Usp18, and Trim30α, which balance the level of cell survival genes to regulate vessel pruning in the retina. Canonical Wnt signaling receptors, coreceptors and ligands cooperate with Dll4/Notch signaling, and pericytes secrete Ang II to balance the progress of vessel remodeling. Canonical Norrin/Fz4/Lrp5/6 accelerate β-catenin nuclear translocation and control the transcription of Cyclin D1 or Myc-Cdkn1a to regulate cell survival. The negative regulatory factor Apcdd1 controls vessel density transiently in retina during P10-12. Dll4/Notch signaling stimulates expression of Nrarp and contributes to canonical Wnt signaling by interacting with Lef1/Ctnnb1. Ang2 produced by pericytes has a dual identity in the regulation of cell death. On the one hand, Ang2 suppresses Akt to permit cell death. On the other hand, Ang2 promotes the secretion of Wnt7b by macrophages to activate the Wnt/β-catenin pathway, which inhibits cell death by promoting cell cycle entry to regulate hyaloid regression