Fig. 12.
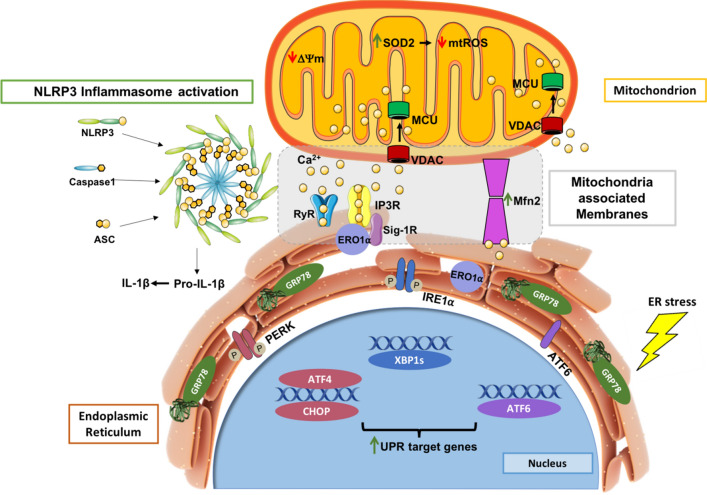
ER-mitochondria communication is implicated in ER stress-induced NLRP3 inflammasome activation in the innate immune system. ER stress modulates the structure of mitochondria-associated membranes (MAMs) in human monocytes as demonstrated by the upregulation of chaperones that reside or that are translocated to these ER-mitochondria close contacts under severe/prolonged ER stress, and by the increment of the Mfn2 ER-mitochondria tethering protein. The proximity between both organelles occurs concomitantly with an early Ca2+ influx into mitochondria and depolarization of mitochondrial membrane, culminating in activation of the NLRP3 inflammasome and release of pro-inflammatory IL-1β that is also affected by the release of Ca2+ from the ER. Enhanced mitochondrial fusion to stimulate energy production can lead to the accumulation of dysfunctional mitochondrial compromising cell viability. Activation of antioxidant defenses within mitochondria to avoid ROS accumulation might represent a protective strategy that is not able to counteract chronic ER stress-induced sterile inflammation