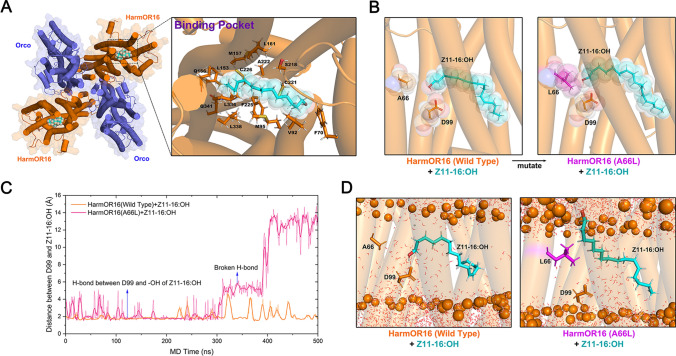
Fig. 7.
Molecular dynamics simulations of substrate-bound HarmOR16. A Structure of the HarmOR16-Z11-16:OH complex after MD relaxation. HarmOR16 and Orco subunits are colored blue and orange, respectively. Z11-16:OH is represented by spheres. The binding pocket is zoomed-in to show the residues surrounding Z11-16:OH. B The A66L mutation of HarmOR16 would cause steric clashes with the bound Z11-16:OH. C MD trajectories of the distance between the sidechain oxygen atom of Asp 99 and the hydroxyl radical of Z11-16:OH (wild-type in red, A66L in blue). D Representative structures to show typical substrate binding modes of the wide-type HarmOR16 and its A66L mutant observed in MD simulations. The phosphate headgroups of POPC are shown as orange spheres and the transmembrane helices of HarmOR16 are represented by transparent cylinders. The hydrogen bonding interaction with Z11-16:OH was quickly broken in the mutant