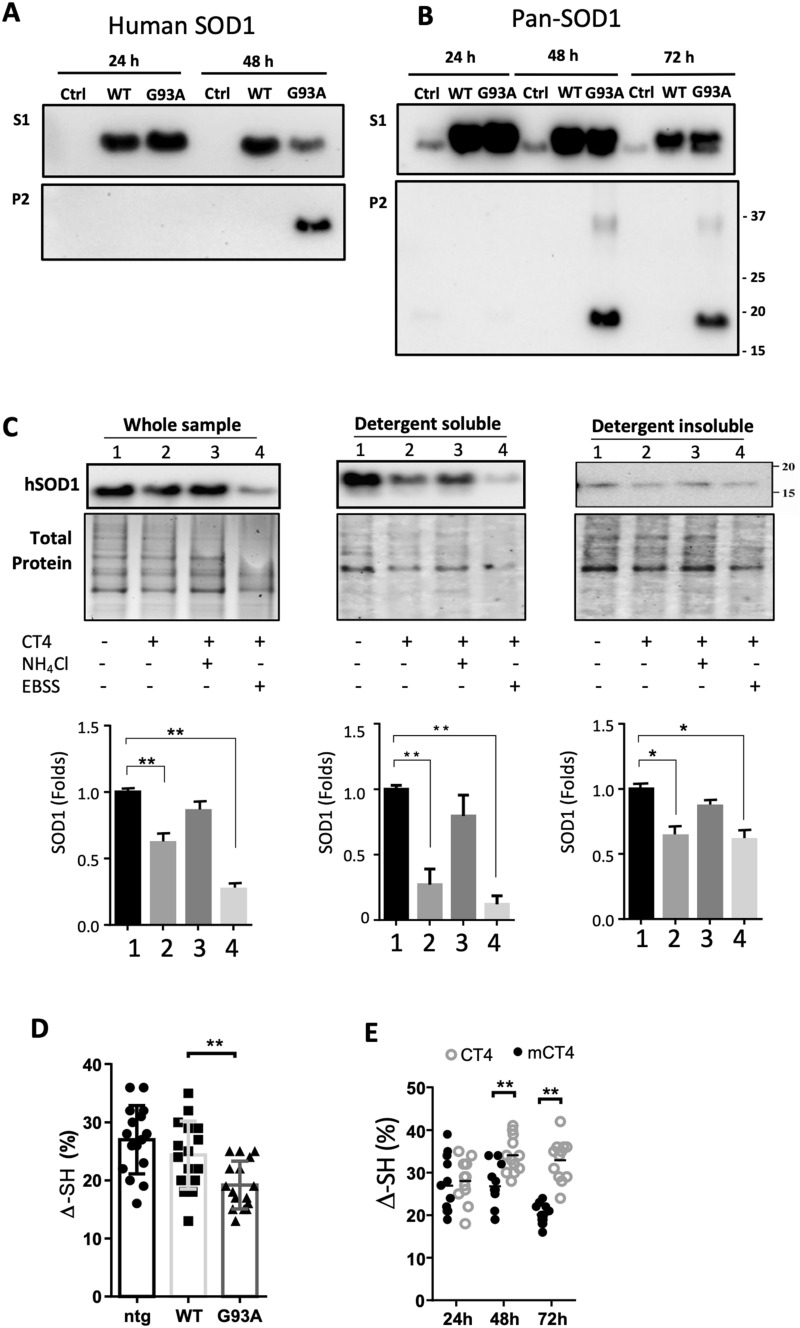
Fig. 3.
CT4 removes misfolded SOD1 in detergent-soluble fraction through lysosomes. a, b ALS-linked G93A-hSOD1 is detectable in both detergent-insoluble (P2) and soluble (S1) fractions. CHO cells were transfected with constructs of WT-hSOD1 and G93A-hSOD1 for indicated hours. Blots were probed with the antibody that recognizes only hSOD1 (a) or pan-SOD1 antibody recognizing both human and hamster SOD1 (b). c Neurons differentiated from embryonic NSCs of the G93A-hSOD1 mice were pretreated with or without 10 mM NH4Cl or EBSS for 1 h, followed by treatment with 5 µM CT4 for 3 h. After treatment, total cellular extracts, detergent-soluble and insoluble fractions from cells were prepared. Equal volumes of lysates with a total of 10 µg protein were loaded to 12% TGX stain-free polyacrylamide gels. Total protein generated by stain-free visualization was used as loading controls. Data on SOD1 expression were analyzed with one-way ANOVA followed by Bonferroni’s multiple comparison post-test. **P < 0.01. Bars represent mean ± SD. n = 3. d A modified immunosorbent assay was used to detect the thiol-disulfide status of the SOD1 in non-transgenic, WT-hSOD1, and G93A-hSOD1 cell lysates. The thiol content was normalized to total hSOD1, measured using a commercial ELISA kit. One-way ANOVA followed by Bonferroni’s multiple comparison post-test was performed to establish significant differences with the non-transgenic (ntg) group: *P < 0.05. Bars represent mean ± SD, n = 6 from 3 separate cultures. e The aerobic/anaerobic ratio was measured by incubating neuron lysate in an aerobic chamber overnight, and anaerobic preserved aliquots were used as references. Two-way ANOVA followed by Sidak’s multiple comparisons test was performed. **P < 0.01. n = 6 from 3 separate cultures