Fig. 2.
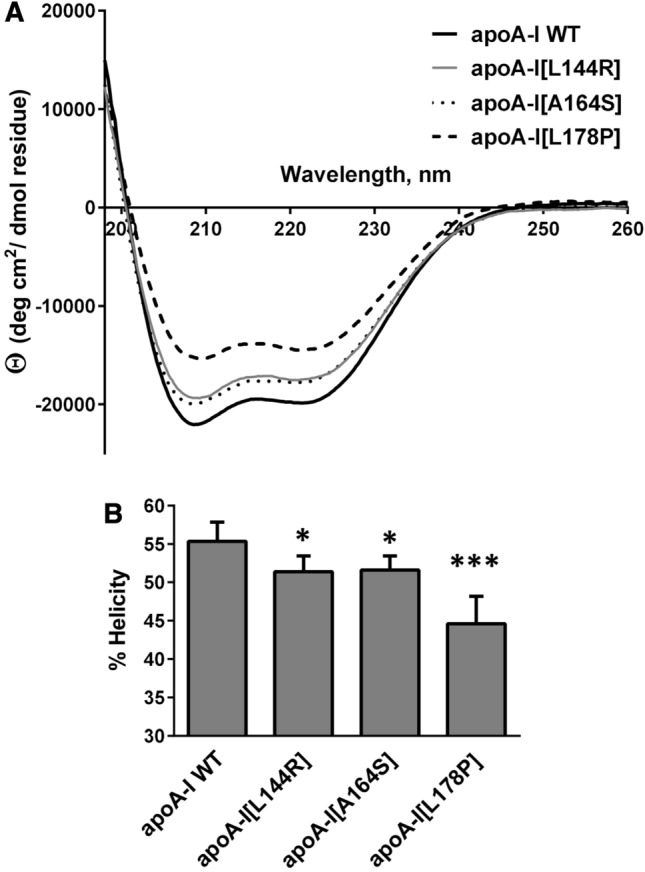
Effect of L144R, A164S and L178P mutations on the secondary structure of apoA-I. a Far-UV CD spectra of WT and mutant apoA-I forms. Spectra are averages of four independent analyses of separate protein preparations. b Percent α-helical content of WT and mutant apoA-I forms. Values represent the means ± SD (n = 4). *p < 0.05; ***p < 0.0001 versus apoA-I WT
