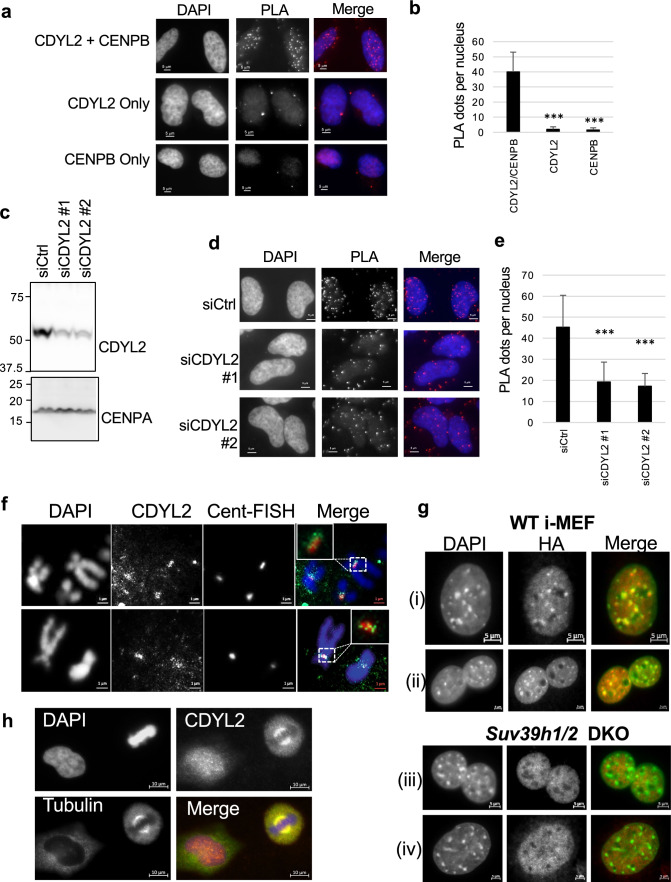
Fig. 4.
CDYL2 colocalization with pericentromeres and the mitotic spindle. a Proximity ligation assay (PLA) of CDYL2 and CENP-B, with concomitant DAPI nuclear staining. Single antibody PLA controls are also shown. Representative images are shown. The experiment was repeated three times with similar results. b Quantification of the PLA dots in the respective conditions tested in (a). The mean number of dots per nucleus and S.D. are shown. T tests comparing single antibody controls to anti-CDYL2/CENP-B PLA were performed (***p < 0.001). c Immunoblot validation of CDYL2 siRNA knockdown corresponding to PLA in (d). d PLA of CDYL2 and CENP-B in Hela cells treated with control siRNA or two independent siCDYL2 sequences, performed as in (a). e Quantification of PLA dots from (d) was performed as in (b). f IF-FISH showing DAPI-stained metaphase chromosomes, immunostained CDYL2, and centromere repeats stained with a labeled FISH probe (Cent-FISH). Two representative images are shown. The white boxes in the merged images indicate a zoom on the co-stained regions. The staining was repeated three times with similar results. g Wild-type (WT) or Suv39h1/2 DKO i-MEF were transduced with HA-mCdyl2 expression vectors (i, iii) or HA-mCdyl1 expression vectors (ii, iv), then stained with anti-HA antibodies (red) and DAPI, shown in green to facilitate analysis of co-localization analysis in the merged images. Shown are representative images from three independent experiments. h CDYL2 and gamma-tubulin IF in Hela cells fixed with methanol. Shown is a representative image of a metaphase cell (right of frame) and interphase cell (left of frame). Staining was repeated three times with similar results