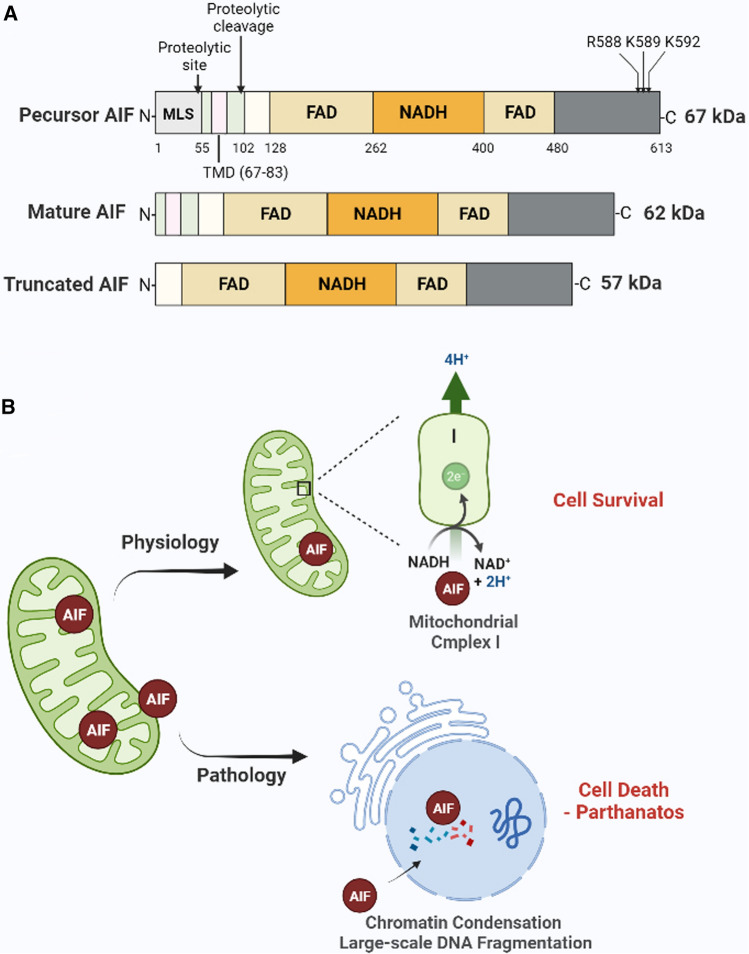
Fig. 3.
Structure and function of AIF. A The structure of the precursor AIF, mature AIF, and truncated AIF. AIF contains three domains: (1) an N-terminal FAD-binding domain (AA: 128–162 and 400–480), (2) a central reduced NAD(NADH)-binding domain, and (3) a C-terminal domain. The precursor AIF is cleaved at the proteolytic site to form mature AIF. In response to the death signal, the mature AIF is further cleaved at the proteolytic cleavage site to form soluble truncated AIF. R588, K589, and K592 are the binding sites of AIF with PAR. B The function of AIF. Under physiological conditions, AIF spans the intermitochondrial membrane and plays a role in stabilizing mitochondrial complex I and maintaining mitochondrial structure. However, in response to death signals (pathology), AIF translocates from the mitochondria to the nucleus causing chromatin condensation and degradation of large DNA fragments, which leads to parthanatos. AIF apoptosis-inducing factor, MIF migration inhibitory factor, PAR poly(ADP-ribose), MLS mitochondrial localization signal, TMD terminal transmembrane domain