Abstract
During macroautophagy, the Atg8 protein is conjugated to phosphatidylethanolamine (PE) in autophagic membranes. In Apicomplexan parasites, two cysteine proteases, Atg4 and ovarian tumor unit (Otu), have been identified to delipidate Atg8 to release this protein from membranes. Here, we investigated the role of cysteine proteases in Atg8 conjugation and deconjugation and found that the Plasmodium parasite consists of both activities. We successfully disrupted the genes individually; however, simultaneously, they were refractory to deletion and essential for parasite survival. Mutants lacking Atg4 and Otu showed normal blood and mosquito stage development. All mice infected with Otu KO sporozoites became patent; however, Atg4 KO sporozoites either failed to establish blood infection or showed delayed patency. Through in vitro and in vivo analysis, we found that Atg4 KO sporozoites invade and normally develop into early liver stages. However, nuclear and organelle differentiation was severely hampered during late stages and failed to mature into hepatic merozoites. We found a higher level of Atg8 in Atg4 KO parasites, and the deconjugation of Atg8 was hampered. We confirmed Otu localization on the apicoplast; however, parasites lacking Otu showed no visible developmental defects. Our data suggest that Atg4 is the primary deconjugating enzyme and that Otu cannot replace its function completely because it cleaves the peptide bond at the N-terminal side of glycine, thereby irreversibly inactivating Atg8 during its recycling. These findings highlight a role for the Atg8 deconjugation pathway in organelle biogenesis and maintenance of the homeostatic cellular balance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-023-05004-2.
Keywords: ATG4, Otu, Apicoplast, Autophagy, Plasmodium, Malaria, Sporozoites, EEF, Cysteine protease, Deconjugation
Introduction
Autophagy (macroautophagy) is a catabolic process conserved from yeast to mammals. Autophagy is induced by many cellular stresses, such as nutrient deprivation, parasitic infection, disease, and differentiation, and acts to maintain cellular homeostasis [1–4]. Autophagy involves the sequestration of cytosolic proteins and often organelles around single membrane-bound structures called isolation membranes, which later expand and mature to form double membrane-bound structures called autophagosomes [5]. The autophagosomes then fuse and deliver their contents to lysosomes, which are degraded by lytic enzymes. The autophagy pathway is regulated by a set of genes called autophagy-related genes (ATGs), and to date, 41 ATGs have been identified [6, 7]. This includes the genes that regulate autophagosome formation, which requires Atg5–Atg12 and the Atg8 conjugation system, as well as other genes required at other steps of autophagy [8, 9]. Atg8 localizes on isolated membranes and autophagosomes and has been considered a marker of the induction and progression of autophagy [10]. The presence of Atg8 on the autophagosome is related to its many roles, such as cargo selection, fusion, and membrane tethering and expansion [11, 12]. In yeast, Atg8 is synthesized with a terminal peptide, which is immediately cleaved by the cysteine protease autophagy-related protein 4 (Atg4), revealing a glycine at the C-terminus, which then acts as a substrate in the ubiquitin-like conjugation reaction mediated by Atg7 (E1-activating enzyme) and Atg3 (E2 conjugating enzyme), which mediates its conjugation to PE on the autophagosome membrane. The conjugation of Atg8 to the membrane is reversible, and Atg8 is deconjugated from the outer surface upon autophagosome closure [13]. Deconjugation is important for replenishing Atg8 for further autophagosome formation [14]. Two different classes of cysteine proteases have been found to play a role in deconjugation: one is Atg4 of the C4 cysteine protease family, which cleaves at the C-terminus of the Atg8–PE bond, and the other is ovarian tumor unit (Otu) of the DUB family, which irreversibly inactivates Atg8 by cleaving at the N-terminus of the Atg8–PE bond [15].
Plasmodium, the causative agent of malaria, is an apicomplexan parasite that alternates its life cycle in mammalian and mosquito hosts. The parasite changes its morphology at every stage of its infection cycle and removes the superfluous organelles. In that light, autophagy-like machinery seems to play a crucial role. However, looking at the redundant set of ATGs in Plasmodium, it is difficult to predict the pathway [16–19]. Additionally, apicomplexan parasites lack autophagosome-like structures, which suggests diversifying their ways to eliminate superfluous organelles. The most conserved part of the autophagy machinery in protist parasites is the ATG8 membrane conjugation system. Additionally, Atg8 in apicomplexan parasites has an exposed terminal glycine, which enables its immediate conjugation to PE without needing cleavage by Atg4 [16, 19]. Parasites lack autophagosome-like structures, and the identified function of autophagy machinery is Atg8 conjugation to PE on the membrane of the apicoplast [19–22]. The conjugation of Atg8 to the apicoplast membrane was shown to be essential for the maintenance and biogenesis of organelles in asexual blood stages of Plasmodium falciparum [23] as well as in the replicative phase of tachyzoite formation in Toxoplasma gondii [24]. The downregulation of Atg8 and the enzymes of the conjugation pathway severely affected parasite propagation [25–28].
All previous studies have shown that the conjugation of Atg8 to the membrane is important for parasite survival. However, its deconjugation and replenishment in parasite survival is not clear. The autophagy pathway supports parasite replication in the host by conjugating Atg8 on the apicoplast membrane and enabling its expansion and division. In light of this, we tried to elucidate the role of the deconjugating enzymes in the Plasmodium berghei life cycle. The previous genome analysis of Plasmodium revealed the conservation of cysteine proteases, specifically known for their role in deconjugating Atg8 from their membrane counterparts.
In this study, we investigated the role of two enzymes, namely, Atg4 and Otu domain-like cysteine proteases. We successfully disrupted both genes individually; however, simultaneously, they were refractory to deletion and essential for parasite survival. Deletion of Atg4 had a more dramatic effect during liver stage development than did deletion of Otu. Our data suggest that Atg4 is the primary deconjugating enzyme, which is critical for parasite development in the liver.
Materials and methods
Parasites and cell lines
Plasmodium berghei ANKA (MRA 311) was obtained from BEI resources, USA. Plasmodium sporozoites were obtained by infecting female Anopheles stephensi mosquitoes reared at the CSIR-Central Drug Research Institute as described previously [29]. Human liver hepatocellular carcinoma (HepG2) cells (ATCC) were regularly maintained in DMEM supplemented with 10% FBS (Biological Industries, 04-121-1A), 0.2% NaHCO3 (Sigma-Aldrich, S5761), 1% sodium pyruvate (CELLclone, CC4016.010L), and 1% penicillin‒streptomycin (Thermo Fisher Scientific, 015140-122) at 37 °C with 5% CO2.
Mice
Female Swiss albino mice (6–8 weeks old) were used for routine parasite infections, and C57BL/6 mice 6–8 weeks old were used for sporozoite in vivo infectivity. All animal procedures were approved by the Institutional Animal Ethics Committee at CSIR-Central Drug Research Institute, India (IAEC/2018/3).
Generation of knockout and transgenic parasites
To generate Atg4 (PBANKA_1025400) knockout parasites, double-crossover homologous recombination was used. For this, two fragments, F1 (967 bp) and F2 (556 bp), were amplified using primer sets 1655/1656 and 1333/1355 from the 5′ and 3′ UTRs of the gene, respectively, and were sequentially cloned into the pBC–GFP–hDHFR vector at XhoI/ClaI and NotI/AscI, respectively. The plasmid was linearized using XhoI/AscI and transfected into P. berghei ANKA schizonts as described previously [30]. Similarly, for the generation of Otu (PBANKA_0515350) knockout parasites, two fragments, F1 (591 bp) and F2 (546 bp), were amplified using primer sets 1964/1965 and 1966/1967 from the 5′ and 3′ UTRs, respectively, and sequentially cloned into the pBC–mCherry–TgDHFR vector at KpnI/ClaI and NotI/AscI, respectively. The plasmid was linearized using KpnI/AscI and transfected into P. berghei ANKA as described above. For the endogenous tagging of Atg8 (PBANKA_0504100) to generate Atg8-3XHA-mCherry transgenic parasites, two fragments, F1 (630 bp) and F2 (547 bp), were amplified using primers 1560/1561 and 1562/1563, respectively, and cloned sequentially into the pBC–3XHA–mCherry–hDHFR vector at XhoI/BglII and NotI/AscI, respectively. The plasmid was linearized using XhoI/AscI and transfected into P. berghei ANKA as described above. All transfected parasites were selected by oral administration of pyrimethamine drug (Sigma-Aldrich, 46706), and resistant parasites were collected and genotyped using 5′ and 3′ site-specific integration PCR using primers 1486/1225 and 1215/1355 for Atg4 KO, 1988/1225 and 1913/1989 for Otu KO and 2020/1218 and 1215/2021 for Atg8-3XHA-mCherry transgenic parasites. All primer sequences are given in Table S1. Clonal lines of the KO and transgenic parasites were obtained by limiting dilution of the parasites in Swiss mice. The otu-3XHA tagging plasmid was obtained from PlasmoGEM resource [31, 32]. The plasmid was linearized using NotI and transfected into P. berghei schizonts as described above, and integration at the locus was confirmed by genotyping using the primer pair 2019/2036.
Generation of Atg4/Otu double KO parasites
For the generation of Atg4/Otu double knockout parasites, the linearized targeting cassette of Atg4 with GFP reporter and hDHFR selection marker was transfected into the purified schizonts of Otu KO parasites with mCherry reporter and TgDHFR selection marker. The transfected parasites were selected by the subcutaneous injection of WR drug (Sigma-Aldrich, SML2976) for 3 consecutive days. Giemsa-stained blood smears were prepared until day 15 to observe the appearance of the double knockout parasites in the blood. In the second approach, Otu–mCherry–TgDHFR and Atg4–GFP–hDHFR linearized cassettes were cotransfected into P. berghei ANKA schizonts. The transfected parasites were selected by the oral administration of pyrimethamine, and the appearance of the resistant parasites was observed by making a Giemsa-stained blood smear.
Cysteine protease activity and delipidation assay
To demonstrate the inherent cysteine proteolytic activity of the deconjugating enzymes, we performed a cysteine protease inhibition assay. A schizont culture of Atg8-3XHA-mCherry transgenic and P. berghei ANKA parasites was set up and grown for 7 h. One hundred microliters of culture was aliquoted in the well of a 96-well plate and treated with the cysteine protease inhibitor E64 (Sigma-Aldrich, E3132). The culture was grown for 14 h at 80 rpm in a 37 °C incubator. After incubation, the culture was pelleted and spotted on a microscopic slide for further immunofluorescence assay analysis. To show the delipidation activity of Atg4, the catalytic domain of Atg4 was expressed in bacteria. The P. berghei codon-optimized Atg4 gene (amino acids 721–967) encoding the catalytic domain was cloned into the pET28a vector, and the culture was induced using 0.2 mM IPTG. The protein was purified using Ni–NTA resin as described previously [33]. To prepare the membrane fraction, iRBCs were treated with 0.15% saponin (Sigma-Aldrich, S4521) and washed three times in PBS. Next, the parasite pellet was lysed by freezing/thawing three times in MSE buffer [225 mM mannitol, 75 mM sucrose, 0.1 mM EDTA, and 3 mM Tris–HCl (pH 7.4)]. The cell debris was separated by centrifugation at 800×g for 5 min. The supernatant was centrifuged at 13,000 ×g for 15 min, and the low-speed fraction was used for the assay. The purified Atg4 was incubated with the membrane fraction with and without E64 inhibitor, and signals were detected using anti-Atg8 antibody.
Western blotting
To visualize the Atg8–PE-conjugated and nonconjugated forms, the low-speed fraction of WT or Atg4 KO lysates was prepared, and the low-speed fraction was solubilized in 1X laemmli buffer (Bio-Rad, 1610747) and separated by 4–20% gradient SDS-PAGE. The proteins were transferred to PVDF membranes (Bio-Rad, 1620177) by electroblotting. The membrane was blocked with 1% BSA–PBS for 1 h at room temperature, followed by incubation overnight at 4 °C with anti-Atg8 antibody (diluted 1:1000) in 1% BSA–PBST (0.1% Tween 20). The membrane was washed three times with 1X PBST and incubated with HRP-conjugated anti-rat antibody (diluted 1:5000, Invitrogen, A18865) for 1 h at RT. The membrane was washed three times with 1× PBST and developed using ECL Chemiluminescent Substrate (Bio-Rad, 170–5060), and signals were detected using a ChemiDoc XRS + System (Bio-Rad). To estimate the equal loading of the parasite lysate, the membrane was probed with HRP-conjugated anti-β-actin antibody (diluted 1:10,000, Sigma, A3854).
Parasite asexual growth and mosquito infection
To analyze whether deleting the genes in the blood stage affected asexual blood-stage propagation, an equal number of iRBCs of knockout and WT control parasites were intravenously injected into Swiss mice. The growth was monitored daily by making Giemsa-stained blood smears. Female Anopheles mosquitoes were allowed to feed on Swiss mice infected with knockout or WT parasites for transmission. On day 14 postfeeding, the mosquito midgut was dissected and imaged under a fluorescence microscope (Nikon Eclipse 80i) using a 10× [numerical aperture (NA), 0.25; air] objective with no filter, and the sporulation pattern and oocyst numbers were determined. Another batch of midguts was crushed, and sporozoites were counted using a hemocytometer. On day 21, salivary glands were dissected, and the sporozoite numbers were enumerated as described previously [34].
In vivo infectivity of sporozoites
To assess in vivo infectivity, 5000 sporozoites were intravenously injected into C57BL/6 mice (5 mice/group). The appearance of parasites in blood was determined by making Giemsa-stained blood smears. To estimate the liver stage parasite biomass, another group of C57BL/6 mice (5 mice/group) was similarly inoculated with sporozoites, and the liver was harvested and homogenized at different time points in RNA-Xpress reagent (HiMedia, MB601). Total RNA was isolated using the manufacturer’s instructions. cDNA was synthesized using total RNA in a reverse transcriptase reaction containing 1× PCR buffer, 0.5 mM dNTPs, 5 mM MgCl2, 20 U RNase inhibitor, 2.5 µM random hexamers and 50 U reverse transcriptase (Applied Biosystems) in a thermocycler (Eppendorf) as previously described [35]. The resulting cDNA was then used for real-time PCR of Pb18s rRNA as described earlier [36] using SYBR premix (Takara Bio, RR420A) with primer sets 1195/1196 and 1219/1220. Mouse GAPDH transcripts were also amplified using primers 1193/1194 to normalize the expression. The transcript number of parasites was ascertained relative to the standard curve of their plasmids and expressed as the normalized values by taking the ratio of Pb18s rRNA/mGAPDH transcripts. All the data were obtained from a CFX Opus 96 real-time PCR system (Bio-Rad), and the primers used for real-time PCR are given in Table S1.
In vitro liver stage development analysis
To visualize liver stage development in vitro, HepG2 cells were infected with sporozoites (5000/well), and the culture was maintained as previously described [37]. The culture was fixed at 24, 36, 48, and 64 hpi by adding 4% PFA and proceeded for IFA. For merosome analysis, 105 HepG2 cells were seeded in a 24-well plate and infected with 30,000 sporozoites. The culture supernatant was collected at 64 hpi and quantified using a hemocytometer. To check merosome infectivity, Swiss mice were i.v. injected with harvested merosomes (10 merosomes/mice). The onset of blood-stage infection was observed by making Giemsa-stained blood smears.
Immunofluorescence assay (IFA)
Treated WT and Atg8-3XHA-mCherry blood-stage parasites were fixed with 4% paraformaldehyde (Sigma-Aldrich, HT5012) for 20 min at RT and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, T8787) for 10 min at RT. Cells were washed with PBS, blocked with 1% BSA–PBS, and incubated with the anti-Atg8 developed in the rat (diluted 1:200), anti-mCherry (diluted 1:500, Novus, NBP 2-25157) and heat shock protein 70 (anti-Hsp70) [38] (diluted 1:500) antibodies for 1–2 h in a blocking solution. Similarly, the infected HepG2 culture was fixed, blocked, and incubated with primary antibodies as described above. To analyze the numbers and area, GFP- or mCherry-expressing EEFs were stained with upregulated in infectious sporozoites gene 4 (UIS4) [39] (diluted 1:1000). The EEFs were counted under a fluorescence microscope (Nikon Eclipse 80i), and the area of the EEFs was measured using NIS-D software. Heat Shock Protein70 (Hsp70) [38] (diluted 1:500) was used to visualize the parasite cytosol, Merozoite formation was visualized using Merozoite Surface Protein 1 (anti-MSP1) antibody [40] (diluted 1:5000), and apicoplast, ER and mitochondria development were visualized using Acyl Carrier Protein (ACP) [41] (diluted 1:1000), anti-Bip (diluted 1:500, BEI Resources, MRA-1247), and immature colon carcinoma transcript-1 (ICT1) [42] (diluted 1:100) antibodies, respectively. To visualize and study the pattern of microneme exocytosis, EEFs were fixed at different time points and immunolabeled with a thrombospondin-related anonymous protein (anti-TRAP) antibody (diluted 1:200). Affinity-purified polyclonal rabbit antibody against P. berghei TRAP was developed by GenScript Inc., Piscataway, NJ, against the peptide sequence CAEPAKPAEPAEPAE. The signals were revealed by their respective Alexa Fluor-conjugated secondary antibodies (diluted 1:1000, Invitrogen). Nuclei were stained with Hoechst 33342 (Sigma-Aldrich, 41399), and the coverslips were mounted using Prolong Diamond antifade reagent (Invitrogen, P36970). The images of asexual blood-stage and mosquito-stage parasites were acquired from a Leica DM 3000 LED microscope using 100× (NA 1.25, oil), 40× (NA 0.65, air) or 10× (NA 0.25, air) objectives. Images of merosomes stained with MSP1, ACP and Bip were acquired using FV1000 software on a confocal laser scanning microscope (Olympus BX61WI) using a UPlanSAPO 100× (NA 1.4, oil) objective. Representative images of EEFs stained with anti-UIS4/TRAP were acquired using a Leica-SP8 confocal microscope (Leica, Wetzlar, Germany) under a 100× oil immersion objective. All images were processed using ImageJ Fiji software and deconvoluted using the “Deconvolution Lab2” plugin.
Immunolocalization
Transgenic asexual blood-stage parasites expressing Otu–HA smear were fixed using 4% paraformaldehyde at RT for 10 min and permeabilized using 0.1% Triton-X-100 for 10 min at RT. The slides were washed three times with PBS and blocked in 1% BSA-PBS for 1 h at room temperature. Next, the slides were incubated with anti-HA (diluted 1:200 Santa Cruz Biotechnology, sc-7392) and anti-ACP or anti-Hsp70 antibodies for 1–2 h at RT. The slides were washed three times with PBS. The signals of HA, Hsp70, and ACP were revealed using Alexa Fluor 594-conjugated anti-mouse IgG (diluted 1:1000; Invitrogen, A11007), Alexa Fluor 488-conjugated anti-mouse IgG (diluted 1:1000; Invitrogen, A11001) and Alexa Fluor 488-conjugated anti-rabbit IgG (diluted 1:1000; Invitrogen, A11008), respectively. The nuclei were stained with Hoechst 33342 and mounted using Prolong Diamond antifade reagent. Images were acquired with a Leica DM 3000 LED microscope using a 100× (NA 1.25, oil) objective, and colocalization was estimated using the JaCop plugin of ImageJ software.
Statistical analysis
Statistical tests were performed using GraphPad Prism Software. As indicated, the statistical tests used were one-way ANOVA and unpaired Student’s t test. A P value of less than 0.05 was considered significant.
Results
Cysteine proteases are essential for Atg8 processing
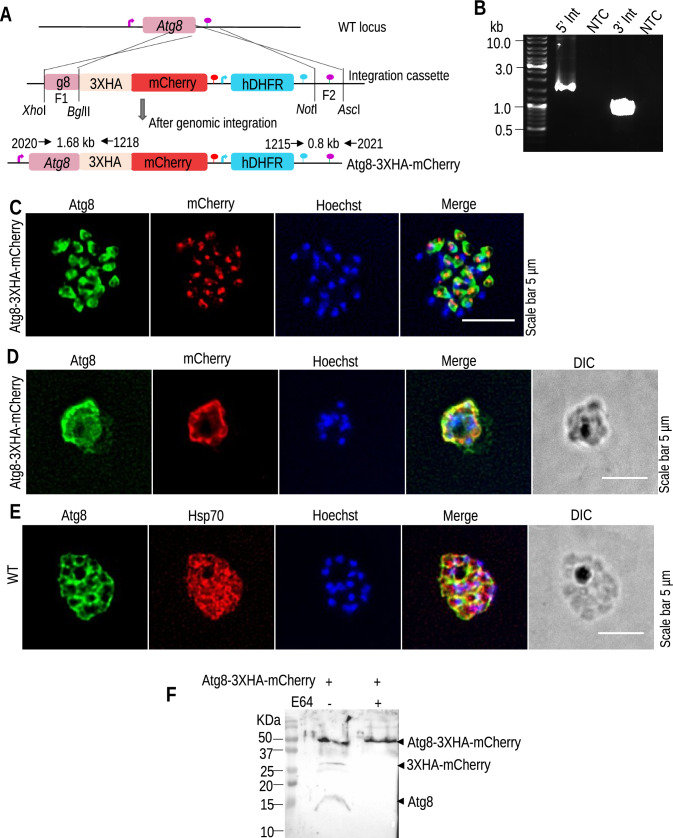
In apicomplexan parasites, Atg8 has a terminally exposed glycine residue that aids its direct conjugation with PE on the apicoplast membrane. To check if Plasmodium Atg4 retains Atg8 processing activity despite its exposed glycine, we investigated the importance of cysteine protease for Atg8 conjugation. Atg8 was endogenously tagged with 3XHA–mCherry at the C-terminus to mask its exposed glycine (Fig. 1A). The targeting cassette was transfected into P. berghei ANKA schizonts, and site-specific integration was confirmed by diagnostic PCR (Fig. 1B). The clonal line was obtained by limiting dilution of the parasites in Swiss mice. Next, Atg8-3XHA-mCherry schizont cultures were treated with the cysteine protease inhibitor E64 to evaluate cysteine protease activity. The treated and untreated schizonts were fixed and analyzed by IFA using anti-Atg8 and anti-mCherry antibodies. Without an inhibitor, we found that mCherry was cleaved, as confirmed by independent signals of Atg8 and mCherry that did not colocalize (Fig. 1C). However, E64-treated Atg8-3XHA-mCherry schizonts showed overlapping staining patterns (Fig. 1D). The treated Atg8-3XHA-mCherry and WT schizonts also failed to properly segregate their nuclei and were found to be arrested during schizogony, which was due to the general inhibition of parasites by E64. Atg8 conjugation was not affected in the arrested WT parasites, suggesting that parasites could conjugate Atg8 with exposed glycine (Fig. 1E). Furthermore, the cleavage of ATG8 was ascertained by western blot analysis of Atg8-3XHA-mCherry schizonts using anti-ATG8 and anti-HA antibodies. We found processing of 3XHA-mCherry in untreated samples, while no processing was observed in E64-treated parasites (Fig. 1F). These results indicate that in the presence of an E64 inhibitor, cysteine proteases failed to process the 3XHA-mCherry tag from the C-terminus of Atg8 and subsequently failed to conjugate on the apicoplast membrane.
Fig. 1.
Atg8-3XHA-mCherry is endogenously processed by cysteine proteases. A Generation of the P. berghei Atg8-3XHA-mCherry transgenic line expressing 3XHA–mCherry under the control of the endogenous Atg8 promoter. B Diagnostic PCR shows correct 5′ and 3′ integrations. C IFA with Atg8-3XHA-mCherry schizonts using anti-Atg8/mCherry antibodies. The distinct localization of Atg8 and mCherry signals suggests that tagged Atg8 was cleaved by cysteine protease. D, E IFA with E64-treated Atg8-3XHA-mCherry and WT schizonts using anti-Atg8/mCherry or anti-Hsp70/mCherry antibodies. E64 treatment resulted in the attenuation of parasites, and both Atg8 and mCherry signals were observed together, suggesting that Atg8-3XHA-mCherry was not cleaved. Nuclei were stained with Hoechst 33342. F Confirmation of Atg8 cleavage by western blot analysis of Atg8-3XHA-mCherry schizonts using anti-ATG8 and anti-HA antibodies. We found processing of 3XHA–mCherry without an inhibitor but not with it
Atg4 is a deconjugating enzyme, and Atg4 KO parasites failed to deconjugate Atg8
To functionally characterize the role of Atg4 in the Plasmodium berghei life cycle, we disrupted the gene using double-crossover homologous recombination, and the successful generation of KO parasites was confirmed by GFP expression and site-specific integration PCR (Fig. S1A–D). Next, we analyzed the asexual blood-stage propagation of Atg4 KO parasites, which was comparable to control WT GFP parasites (Fig. S1E). Upon transmitting the Atg4 KO parasites to female Anopheles mosquitoes, we found normal development in the midgut and salivary glands (Fig. S2A–F). Next, despite its dispensability, we assessed the deconjugation of Atg8 in the Atg4 KO parasite’s blood stages. To visualize Atg8 distribution, IFA was performed with blood-stage parasites using an anti-Atg8 antibody, revealing cytosolic signals of Atg8 in both WT and Atg4 KO parasites. PCC values indicated a significantly higher level of cytosolic Atg8 in the KO parasites, possibly due to its overexpression and accumulation of Otu deconjugated Atg8. (Fig. 2A and B). Furthermore, immunostaining with an anti-ACP antibody revealed Atg8 localization on the apicoplast membrane, which was significantly higher in the Atg4 KO parasites due to impaired deconjugation activity (Fig. 2C and D). Quantification of Atg8 levels in Atg4 KO parasites using western blotting followed by densitometry revealed increased expression of both nonconjugated and conjugated forms of Atg8 in Atg4 KO parasites compared to WT parasites (Fig. 2E–G). This indicated that Atg8 can be conjugated to the membrane due to its exposed C-terminal glycine; however, in the absence of Atg4, parasites failed to replenish Atg8 and overcome its need by overexpressing Atg8. The higher level of deconjugated Atg8 is not only added by its overexpression but also includes the nonrecyclable Otu deconjugated Atg8 form. Next, we analyzed the mitochondrial network in Atg4 KO parasites in asexual blood stages. We found normal differentiation and expansion contrary to a report showing fragmented mitochondria in TgAtg4 KO parasites (Fig. 2H) [27]. To show Atg4 deconjugating activity, we expressed recombinant Atg4 in bacteria (Fig. S3). Purified Atg4 was incubated with a membrane fraction of parasites with or without E64, and we found that Atg4 delipidated Atg8 from the membrane (Fig. 2I). Altogether, these results show that Atg4 activity is important for Atg8 deconjugation in Atg4 KO parasites and that this deconjugation defect is compensated by Atg8 overexpression. This Atg8 overexpression seems to be a short-term rescue because our attempt to disrupt Atg4 and Otu simultaneously failed. We observed few parasites expressing GFP and mCherry posttransfection, indicating double gene disruption (Fig. S7). However, double KO parasites failed to survive during multiplication.
Fig. 2.
Atg4 is required for Atg8 deconjugation from the membrane. A IFA of WT and Atg4 KO blood-stage parasites with anti-Hsp70 and anti-Atg8 antibodies. Atg8 and the cytosolic protein marker Hsp70 showed partial colocalization in the WT parasites (PCC = 0.681 of 20 parasites) and Atg4 KO parasites (PCC = 0.875 of 20 parasites). B Individual PCC values of the 20 parasites stained with anti-Hsp70 and anti-Atg8 antibodies indicated a significantly higher level of cytosolic Atg8 in the KO parasites. C IFA of WT (PCC = 0.505 of 20 parasites) and Atg4 KO (PCC = 0.619 of 20 parasites) parasites with anti-ACP and anti-Atg8 antibodies showing higher overlapping of Atg8 with apicoplast in Atg4 KO parasites. D Individual PCC values of the 20 parasites stained with anti-ACP and anti-Atg8 antibodies indicated a significantly higher level of conjugated Atg8 in the KO parasites. E Western blot analysis of Atg4 KO parasites shows a higher Atg8–PE-conjugated and nonconjugated level than WT parasites. F, G Quantification of Atg8 and Atg8-PE western blot bands of WT and Atg4 KO parasites shows an increase in conjugated and nonconjugated Atg8 in Atg4 KO parasites. H Mitochondrial branching in Atg4 KO parasites was comparable to that in WT parasites. I Delipidation of Atg8–PE from purified parasite membrane fraction (Mem fn) using recombinant PbAtg4. The parasite membrane fraction was incubated with recombinant PbAtg4 with or without the E64 inhibitor. The signals were detected by immunoblotting using an anti-Atg8 antibody. We found delipidated Atg8 as a prominent band in the reaction mixture without inhibitor after PbAtg4 activity (lane 1), whereas in the presence of inhibitor, lipidated Atg8 was prominent (lane 2). The control reaction, without PbAtg4, showed only the lipidated form of Atg8 (lane 3)
Atg4 is critical for late liver stage development
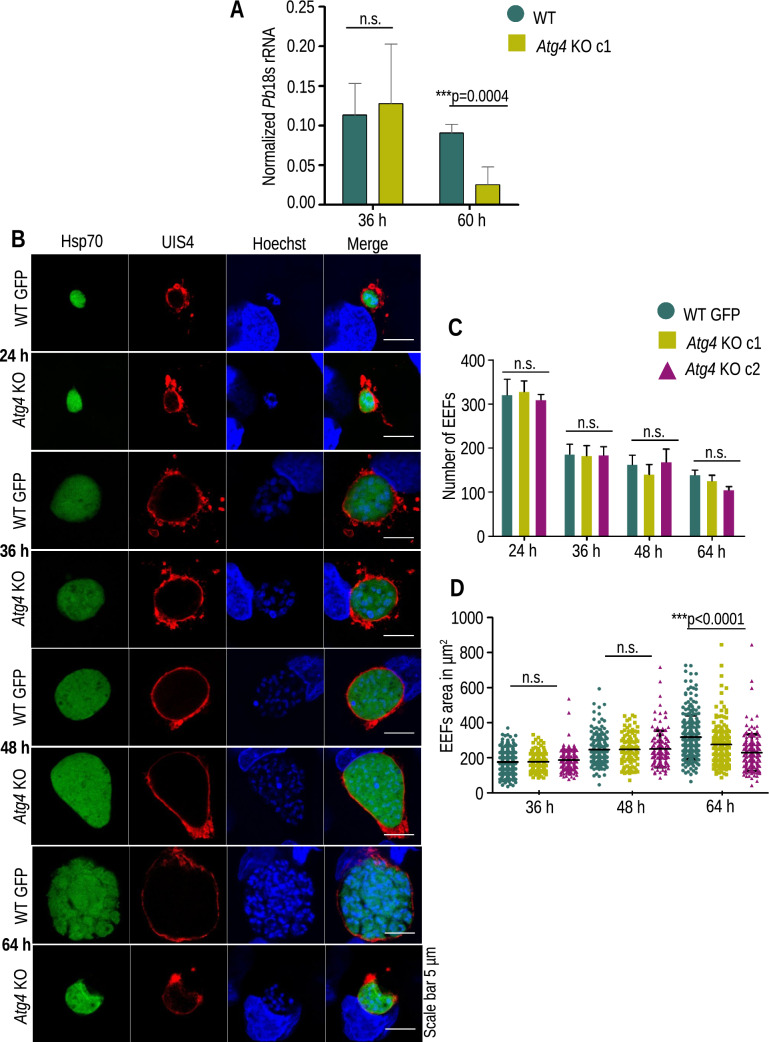
To analyze the in vivo infectivity of Atg4 KO parasites, sporozoites were inoculated intravenously or through mosquito bites in C57BL/6 mice. Mice inoculated with WT sporozoites became patent on day 3 postinfection, whereas the KO group either did not lead to blood-stage infections or showed a delay in patency of 3–8 days (Table 1). To explore whether this compromised sporozoite infectivity occurred due to impaired liver stage development, another group of mice was i.v. injected with 5,000 WT GFP or Atg4 KO sporozoites, and livers were harvested at 36 and 60 hpi. Next, the parasite biomass was quantified using real-time PCR by amplifying Pb18s rRNA. There was no difference in liver parasite burden harvested at 36 hpi; however, it was significantly decreased in Atg4 KO infected livers harvested at 60 hpi compared to WT GFP, indicating the late arrest of Atg4 KO parasites during liver stage development (Fig. 3A). To visualize the developmental defects, HepG2 cells were infected with sporozoites, fixed at different time points and immunostained PVM using anti-UIS4 antibody. We found normal development of EEFs until 48 hpi and then failed to divide further (Fig. 3B). The EEF numbers of Atg4 KO parasites were comparable to those of WT GFP parasites (Fig. 3C). The area of Atg4 KO EEFs was comparable to that of WT GFP parasites until 48 hpi and then found to be smaller when compared at 64 hpi (Fig. 3D). The Atg4 KO EEFs failed to differentiate into nuclei compared to WT GFP parasites. This indicates that the proteolytic activity of Atg4 is required for parasite differentiation at the late time points of development.
Table 1.
Infectivity of WT GFP and Atg4 KO sporozoites in C57BL/6 mice
| Experiment | Parasites | Number of sporozoites inoculated | Mice positive/mice inoculated | Pre-patent period (day) |
|---|---|---|---|---|
| 1 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c1 | 5000 | 2/5 | 5.5 | |
| 2 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c1 | 5000 | 3/5 | 6.3 | |
| 3 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c1 | 5000 | 4/5 | 8.75 | |
| 4 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c2 | 5000 | 5/5 | 5.2 | |
| 5 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c2 | 5000 | 1/2 | 6 | |
| 6 | WT GFP | 5000 | 3/3 | 3 |
| Atg4 KO c2 | 5000 | 0/5 | N/A | |
| 7 | WT GFP | From 20 mosquitos | 5/5 | 3 |
| Atg4 KO c1 | From 20 mosquitos | 4/5 | 8 | |
| 8 | WT GFP | From 20 mosquitos | 5/5 | 3.5 |
| Atg4 KO c2 | From 20 mosquitos | 3/5 | 6.6 |
Salivary gland sporozoites were injected intravenously, and the prepatent period was determined by microscopic examination of Giemsa-stained blood smears. All the mice in the WT group became patent on day 3, while fewer mice became patent in the KO group, with a significant delay in the prepatent period. The chi-square test and significance formula calculated the significance, and the value was found to be 2.29, which is greater than 0.05 (significant level)
Fig. 3.
Atg4 KO parasite development in hepatocytes. A Comparison of Pb18s rRNA transcript numbers in WT GFP- and Atg4 KO-infected mouse livers harvested at 40 and 60 hpi. Representative of two independent experiments is presented as the mean ± SD (no significant difference, at 40 h (p = 0.71458), the significant difference at 55 h (p = 0.004), Student’s t test). B HepG2 cells infected with Atg4 KO and WT sporozoites were fixed at 24, 36, 48, and 64 hpi. The parasites were stained with anti-UIS4 antibody, and nuclei were stained with Hoechst 33342. C Quantification of EEF numbers and data are presented as the mean ± SEM. n = 3 biological replicates (no significant difference, 24 h (p = 0.8762), 36 h (p = 0.9922), 48 h (p = 0.6970) and 64 h (p = 0.1396), one-way ANOVA) D Quantification of EEF area and data presented are pooled from three independent experiments. n = 150–200 EEFs (no significant difference at 36 h (p = 0.1148), 48 h (p = 0.9394), and significant difference at 64 h (p < 0.0001), one-way ANOVA). These results show that Atg4 KO parasites arrest during liver stage development
Atg4 KO parasites show impaired merozoite formation
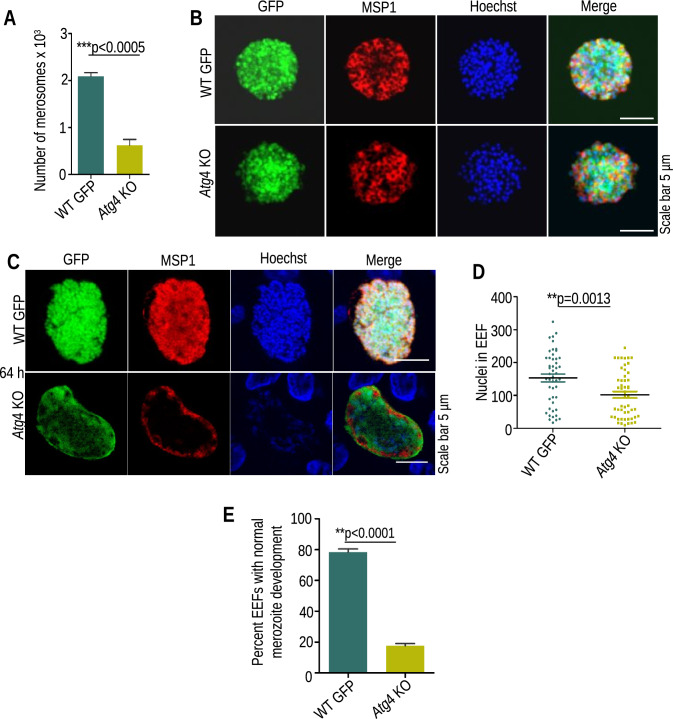
To further identify the stage-specific defect during liver stage development, HepG2 cells were infected with 30,000 sporozoites in 24-well culture plates, and the supernatant containing the detached cells (merosomes) was collected at 64 hpi and counted using a hemocytometer. The number of merosomes was severely reduced in Atg4 KO parasites compared to WT GFP parasites (Fig. 4A). The merosomes were injected into Swiss mice (10 merosomes/mice), and parasitemia was observed by making a Giemsa-stained blood smear. Mice infected with both groups became patent (Table 2), suggesting that few Atg4 KO parasites that matured into merosomes could initiate blood-stage infection. Next, we performed IFA with merosomes and EEFs harvested at 64 hpi using an anti-MSP1 antibody and found that the merosomes had normal MSP1 staining and merozoite formation (Fig. 4B); however, the majority of Atg4 KO EEFs lacked MSP1 staining and had reduced nuclei counts (Fig. 4C–E). The results suggest that Atg4 is critical for late liver stage development.
Fig. 4.
Atg4 KO parasites show impaired merozoite development. A Quantification of merosome numbers collected from the culture supernatant. Data are presented as the mean ± SEM. n = 3 biological replicates, (significant difference (p < .0005), Student’s t test). B Merosomes were stained with anti-MSP1 antibody showing normal merozoite formation. C EEFs fixed at 64 hpi were stained with anti-MSP1 antibody showing impaired merozoite formation in Atg4 KO parasites. D Nuclear quantification of WT and Atg4 KO EEFs harvested at 64 hpi and stained with Hoechst 33342. E Quantification of EEFs with normal MSP1 staining and fully developed merozoites. A total of 75 EEFs were analyzed from three independent experiments (data presented as the mean ± SEM, a significant difference (p < 0.0001), Student’s t test)
Table 2.
Merosome formation is impaired in Atg4 KO parasites
| Experiment | Parasites | Number of merosomes injected | Mice positive/mice injected | Pre patent period (days) |
|---|---|---|---|---|
| 1 | WT | 10 | 5/5 | 5 |
| Atg4 KO | 10 | 5/5 | 5.6 | |
| 2 | WT | 10 | 5/5 | 4 |
| Atg4 KO | 10 | 5/5 | 6.8 |
Swiss mice were injected with merosomes, and the prepatent period was determined by microscopic examination of Giemsa-stained blood smears
Atg4 is dispensable for exocytosis of micronemes
As the sporozoite invades the hepatocyte, unnecessary superfluous organelles are secreted from the parasites into the host cytosol. As described previously [43], we also recently demonstrated that the autophagy pathway is essential for the exocytosis of micronemes [44]. In Plasmodium, Atg8 can be directly conjugated to PE because its terminal glycine is exposed, and Atg4 activity may not be required at this stage. We monitored the exocytosis pattern during liver stage development using the anti-TRAP antibody to rule out the possibility that the Atg4 KO phenotype is due to a lack of deconjugation activity and not because of conjugation. We found that by 24 hpi, micronemes were directed toward the PV membrane of the parasite, and by 40–55 hpi, all the micronemes were excreted out of the parasite cytosol, suggesting normal exocytosis in both the KO and WT parasites (Fig. S4). This confirms that Atg4 activity is not required for the exocytosis of micronemes.
Atg8-PE deconjugation is essential for organelle biogenesis during liver stage development
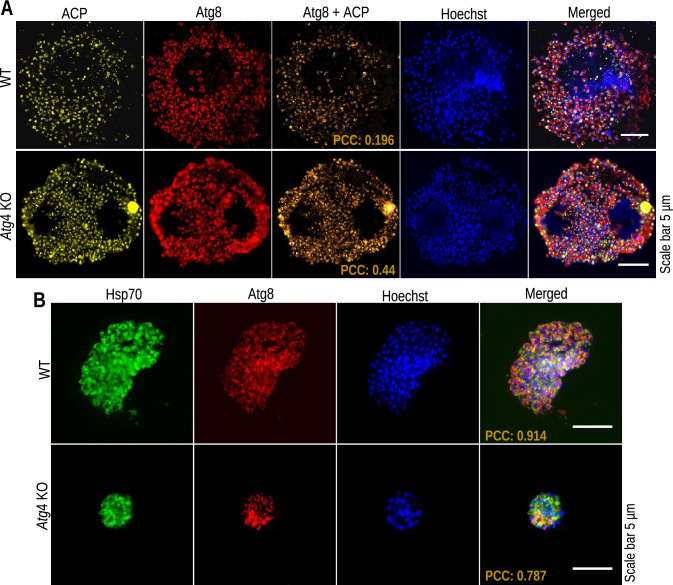
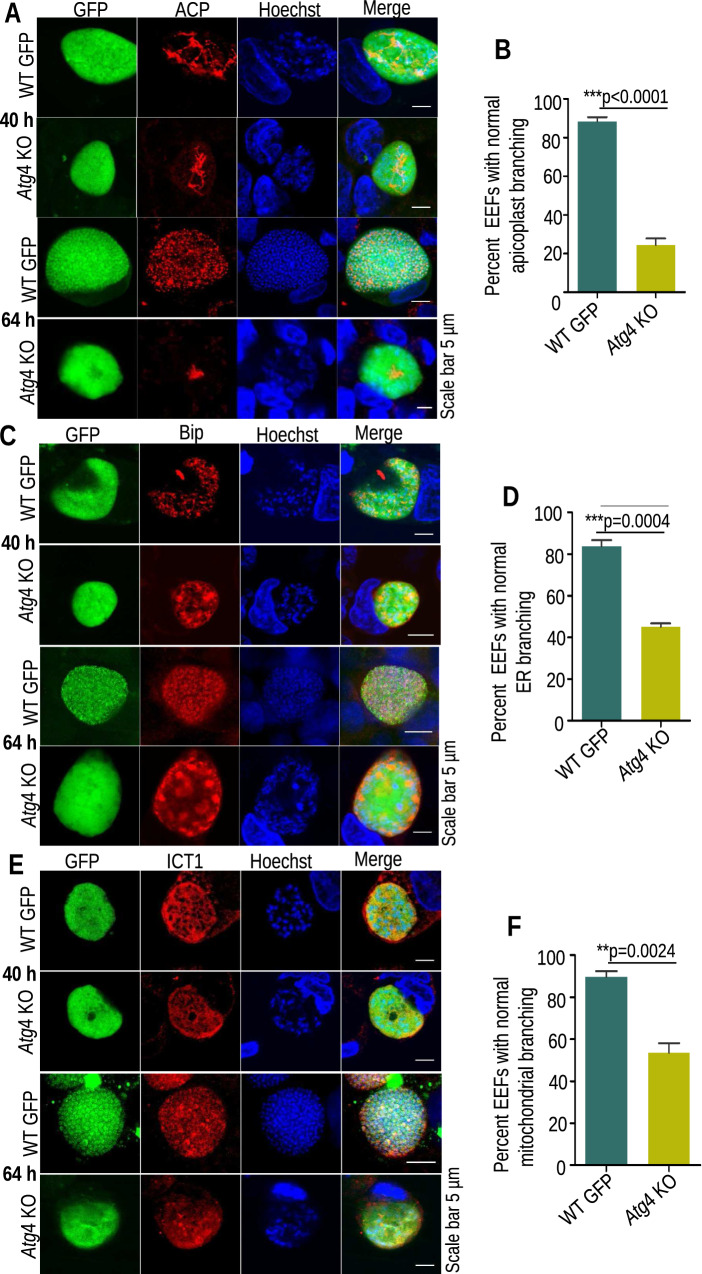
Atg8-PE deconjugation is important for efficient isolation membrane expansion in Saccharomyces cerevisiae [45]. We next investigated the role of Atg4 in the deconjugation of Atg8 from the membrane and how it affects the differentiation of the organelles. For this, KO and WT merosomes harvested at 64 hpi were immunostained with anti-Atg8 and anti-ACP antibodies. We found a significant increase in the localization of Atg8 on the apicoplast membranes in Atg4 KO parasites compared to WT parasites, with PCC values of 0.196 and 0.44, respectively (Fig. 5A). Alternatively, in WT EEFs, a higher level of Atg8 was found to be cytosolic (Fig. 5B). To further illustrate whether this impaired Atg8 deconjugation affects organelle biogenesis, IFA was performed with EEFs harvested at 40 and 64 hpi. We found no difference in organelle branching at 40 hpi, but it was impaired at 64 hpi. The effect was prominent on apicoplast biogenesis compared to mitochondria and the ER (Fig. 6A–F). These results indicate that Atg4 activity is required for the deconjugation of Atg8 from the membrane and further organelle biogenesis.
Fig. 5.
Atg4 KO parasites failed to deconjugate Atg8 from the apicoplast. A Merosomes stained with anti-ACP and anti-Atg8 antibodies show minimum overlapping of signals (orange) in WT parasites (PCC = 0.196); however, there was significantly increased overlapping of signals (orange) in Atg4 KO parasites (PCC = 0.44). B Immunostaining for Atg8 and Hsp70 showed a higher level of Atg8 in the cytosol in WT cells (PCC = 0.911, n = 20) than in KO cells (PCC = 0.8594, n = 20). Nuclei were stained with Hoechst
Fig. 6.
Atg4 KO parasites show impaired organelle differentiation. A, C, E Cultures fixed at 40 and 64 hpi were stained with anti-ACP, anti-Bip, or anti-ICT1 antibodies to visualize apicoplast, ER, or mitochondrial branching, respectively. Organelle branching was similar in WT GFP and Atg4 KO mice at 40 hpi but impaired at 64 hpi. The effect was prominent on apicoplast branching compared to the mitochondria and ER. Nuclei were stained with Hoechst 33342. B, D, F Quantification of EEFs showing normal branching of organelles at 64 hpi in WT and Atg4 KO parasites. A significant number of EEFs showed impaired organelle branching in Atg4 KO parasites. A total of 75 EEFs were analyzed for each organelle from three independent experiments (data presented as the mean ± SEM, a significant difference (p < 0.0001, apicoplast; p = 0.0004, ER; p = 0.0024, mitochondria) Student’s t test
Characterization of Otu in all stages of the parasite life cycle
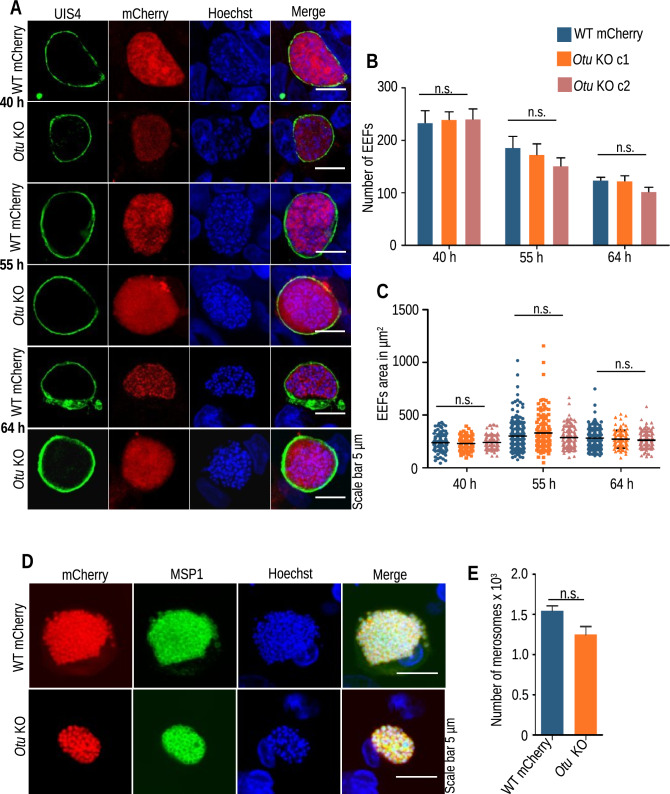
Since the absence of Atg4 did not show any exclusive phenotype, we sought to characterize the role of another cysteine protease, Otu, which was reported to cleave the conjugated forms of Atg8-PE [46]. First, we confirmed a previous report showing apicoplast localization of Otu in P. falciparum. For this, we obtained an Otu-tagged construct from Plasmogem with a hemagglutinin (HA) epitope tag. We transfected P. berghei schizonts, and site-specific integration was confirmed by diagnostic PCR (Fig. 7A and B). Transgenic blood-stage parasites expressing Otu–HA were immunostained with anti-HA/ACP or anti-HA/Hsp70 antibodies. We found apicoplast localization of Otu in the blood stages of Plasmodium parasites (Fig. 7C and D). To investigate the role of Otu in the P. berghei life cycle, we disrupted the gene. For this, the gene’s 5′ and 3′ UTRs were cloned into the pBC–mCherry–TgDHFR vector, and the targeting cassette was transfected into P. berghei ANKA schizonts (Fig. S5A). Resistant mCherry-expressing parasites were observed under a fluorescence microscope, and diagnostic PCR confirmed site-specific integration (Fig. S5B and C). The clonal lines were obtained by limiting dilutions of the parasites, and the absence of ORF was confirmed by PCR (Fig. S5D). The Otu KO parasites propagated normally during asexual blood stages, indicating that the gene is dispensable for blood-stage development (Fig. S5E). Upon transmission to female Anopheles mosquitoes, the Otu KO parasites showed a normal oocyst pattern, which sporulated normally into sporozoites and were successfully transmitted to salivary glands (Fig. S6A–F). Furthermore, we analyzed the in vivo infectivity of Otu KO sporozoites. For this, sporozoites were inoculated i.v. into C57BL/6 mice, and patency was observed by making Giemsa-stained blood smears. The WT parasites established the blood-stage infection on day 3 p.i., whereas, the Otu KO parasites showed a one-day delay in patency (Table 3). Due to a 1-day delay in patency, we performed an in vitro EEF development assay to visualize any defects. For this, HepG2 cells were infected with Otu KO and WT mCherry sporozoites, and their development was observed at 40, 55, and 64 hpi, which was normal (Fig. 8A). To determine whether disruption of the Otu gene affected EEF morphology and number, we quantified the number and area, which were also found to be normal (Fig. 8B and C). To quantitatively assess merozoite development, EEFs harvested at 64 hpi were stained with anti-MSP1 antibody, which showed normal differentiation of the nucleus and merozoites in Otu KO parasites (Fig. 8D). Furthermore, we counted the merosomes released in the culture supernatant in Otu KO parasites, which were comparable to those in WT parasites (Fig. 8E). This indicates that the cysteine protease Otu plays no significant role in parasite development and differentiation during liver stages.
Fig. 7.
Otu localizes to the apicoplast. A Schematic showing the generation of P. berghei Otu–HA transgenic parasites. B Diagnostic PCR confirmed correct integration using primer pair 2036/2019. No band was amplified in WT. C IFA of asexual blood-stage Otu–HA transgenic parasites stained with anti-Hsp70 and anti-HA antibodies. D Otu–HA and the apicoplast protein marker ACP show colocalization in Otu–HA transgenic parasites at the ring (PCC = .0763), trophozoite (PCC = 0.846) and schizont (PCC = 0.593) stages. Nuclei were stained with Hoechst 33342
Table 3.
Infectivity of WT mCherry and Otu KO sporozoites in C57BL/6 mice
| Experiment | Parasites | Number of sporozoites injected | Mice positive/mice injected | Pre patent period (days) |
|---|---|---|---|---|
| 1 | WT mCherry | 5000 | 5/5 | 3 |
| Otu KO c1 | 5000 | 5/5 | 4 | |
| 2 | WT mCherry | 5000 | 5/5 | 3 |
| Otu KO c1 | 5000 | 5/5 | 4 | |
| 3 | WT mCherry | 5000 | 5/5 | 3 |
| Otu KO c2 | 5000 | 5/5 | 4 | |
| 4 | WT mCherry | 5000 | 5/5 | 3 |
| Otu KO c2 | 5000 | 5/5 | 4 |
Salivary gland sporozoites were injected intravenously, and the prepatent period was determined by microscopic examination of Giemsa-stained blood smears
Fig. 8.
Otu KO parasites invade and develop normally into liver stages. A HepG2 cells were infected with Otu KO and WT mCherry sporozoites, and the culture was fixed at 40, 55, and 64 hpi. The PVM was stained with anti-UIS4 antibody, and nuclei were stained with Hoechst 33342. B Determination of EEF numbers (no significant difference at 40 h (p = 0.9607), 55 h (p = 0.4679) and 64 h (p = 0.1850), one-way ANOVA) C Determination of EEF area. Data were pooled from three independent experiments. n = 150–200 EEFs per parasite strain (no significant difference at 40 h (p = 0.6037), 55 h (p = 0.1240) and 64 h (p = 0.1983), one-way ANOVA). D WT and Otu KO EEFs harvested at 64 hpi were immunostained with anti-MSP1. E Normal development of merosomes was observed in the KO parasites. Quantification of the merosomes collected from the culture supernatant showing normal development and egress of merozoites (data presented as the mean ± SEM, n = 3, no significant difference (p = 0.0666), Student’s t test)
Atg4/Otu double gene deletion is lethal for blood-stage propagation
We attempted to disrupt both genes simultaneously to check the redundancy in Atg4 and Otu functions. For this, mCherry-expressing Otu KO schizonts with a TgDHFR selection marker were transfected with GFP-expressing and hDHFR-resistant Atg4-targeting cassettes (Fig. S7A). Transfected parasites were selected by subcutaneous injection of WR drug for 3 days. The generation of Atg4/Otu double KO parasites was visualized by the appearance of parasites expressing both mCherry and GFP; however, none exhibited both signals. Next, we electroporated schizonts with Atg4 and Otu targeting cassettes simultaneously. The transfected parasites were selected by oral administration of the pyrimethamine drug. We observed the parasites daily under a fluorescence microscope and found mostly either GFP- or mCherry-expressing parasites. We also observed rare parasites expressing mCherry and GFP together; however, these parasites were not recovered after drug selection (Fig. S7B). These results suggest that Atg4 and Otu may have an overlapping function during blood-stage development and that they cannot be disrupted together.
Discussion
Maintenance of protein turnover and organelle integrity is important for cell viability. Autophagy is a catabolic process that promotes the recycling of intracellular material and regulates homeostasis in cells; however, its role remains poorly characterized in apicomplexan parasites. In the human malaria parasite P. falciparum, Atg8 localizes to the apicoplast [19], suggesting a role in segregating this essential organelle and protein turnover. We focused our functional studies on deconjugating enzymes of the Atg8 conjugation pathway in P. berghei using reverse genetic approaches. We identified two deconjugating enzymes, Atg4 and Otu, possibly having overlapping functions to liberate Atg8 from the PE-conjugated forms. The absence of these enzymes had no significant role in blood and mosquito stage development; however, the loss of either enzyme affected liver stage development. The loss of Atg4 had a prominent effect on liver stage development compared to Otu. Atg4 was originally identified in yeast as a sole cysteine protease specific to Atg8 [47, 48]. In apicomplexan parasites, with the exposed terminal glycine on Atg8, Atg4 is not responsible for the direct conjugation of Atg8 to the membrane [49]. In addition to activation, Atg4 deconjugates Atg8–PE by cleaving between the C-terminal carboxyl moiety and the amine group of PE. In mammals, deconjugating Atg8 from the membrane is essential for replenishing Atg8 for the next round of conjugation reactions [14] and elongating isolation membranes [45].
The dispensability of the Atg4 gene in the P. berghei asexual blood stages is consistent with a report in P. falciparum where it was found to be dispensable in a genetic screen [50]. Despite its dispensability, Atg4 retains conjugation and deconjugation activity during blood stages and processes 3XHA–mCherry of the tagged Atg8-3XHA-mCherry transgenic parasites. This processing was blocked in the presence of the cysteine protease inhibitor E64. Similarly, the C-terminal end of Atg8 in T. gondii ends with a glycine residue [27]. By constructing GFP–TgAtg8 and site-directed mutagenesis, it was shown that TgAtg4 peptidase activity is required for regulating the conjugation of TgAtg8 [27]. This indicated that Atg4 has an inherent cysteine protease activity. We recently demonstrated that conjugation of Atg8 is essential for eliminating superfluous organelles during liver stage development [44]. However, the elimination of micronemes was unaffected in Atg4 KO parasites because the glycine residue of Atg8 was exposed, which led to the conjugation of Atg8 on the membrane without its processing by Atg4. A significant number of parasites lacking Atg4 failed to differentiate their nuclei and organelles, and possibly those parasites failed to initiate the blood-stage infection. ATG8 has been implicated in both autophagy and apicoplast biogenesis in P. falciparum [22, 23, 51]. Plasmodium autophagy proteins were found to be associated with autophagosome-like vesicles, but how they are used to generate autophagosomes is still enigmatic. The autophagy-related protein PfAtg5 was found to be partially colocalized with ER, mitochondria, apicoplast and PfAtg8 [51]. Depleting either TgAtg3 or TgAtg4 leads to pronounced fragmentation of their mitochondrion [27, 49, 52]. The close apposition and contact sites that exit between the apicoplast and ER are potentially required for the subcellular distribution of lipids in parasites, which explains impaired ER differentiation due to the failure of apicoplast biogenesis [53, 54]. These results further support previous studies indicating the role of ATG8 in apicoplast biogenesis. However, further studies are required to confirm its role in ER and mitochondrial biogenesis.
Atg4 KO parasites showed a severe defect in liver stage development; however, few parasites matured into hepatic merozoites, leading to a blood-stage infection in mice. The mature parasites showed a deconjugated Atg8 form, which suggested that mature parasites were able to deconjugate Atg8 from the membrane and indicated the presence of another deconjugating enzyme. Recently, the deconjugating activity of the ovarian tumor unit (Otu) was shown in P. falciparum blood stages [46]. RavZ/Otu is another cysteine protease of the deubiquitinating enzyme DUB family [55] that has a very specific deconjugating activity toward Atg8–PE. Otu is not evolutionarily related to Atg4 and acts differently than Atg4. First, Otu specifically acts on the PE-conjugated forms and has very little activity toward the precursor forms, and second, Otu acts on the peptide bond at the N-terminal side of the C-terminal glycine, which irreversibly inactivates Atg8 that cannot be conjugated further [15]. On characterizing the role of Otu at various stages of the parasite’s life cycle, we observed a one-day delay in the appearance of parasites in the blood. We found no visible defects in parasite schizogony or merozoite differentiation upon observing the liver stage forms at different time points. Our results are supported by the high-throughput reverse genetic screening in P. berghei [56] and P. falciparum[50] and phenotyping results on Otu in P. berghei; however, they did not observe any defect throughout the parasite life cycle stages [57]. In contrast, the knockdown of Otu in P. falciparum resulted in abnormal apicoplast development and impaired growth of parasites [46]. They also observed the role of PfOtu in the deconjugation of Atg8. These results suggest that Atg4 and Otu both perform deconjugating functions in P. berghei and that the activity of both enzymes is required for normal life cycle completion. However, it appears that Atg4 is the primary deconjugating enzyme, and Otu cannot replace its function completely.
Since we obtained mixed results, we tried to generate Atg4/Otu double KO parasites lacking both deconjugating enzymes. For a very transient phase after transfection, we observed parasites expressing both GFP and mCherry, indicating double KO, but with time, we lost that population, and the parasite population with single gene deletion survived. Additionally, the frequency of parasites lacking Otu was more prominent than that of Atg4 KO parasites. This indicates that both proteins might have an overlapping function in deconjugating Atg8 from the membrane. Otu compensated the absence of Atg4 in a way that deconjugates Atg8 but does not replenish it for further use. The lack of the conjugable forms of Atg8 was compensated by overexpressing Atg8, increasing the overall concentration of Atg8 in the parasites. Therefore, in the absence of Otu, the compensation of Atg8 by Atg4 is more useful; however, the absence of Atg4 was not fully fulfilled by Otu. This explains why the Otu KO parasites completed the cycle without any major defect; however, the Atg4 KO parasites showed attenuation in liver stages. Overall, the study shows that the deconjugation pathway is essential for parasite survival.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Kota Arun Kumar (University of Hyderabad, India) for the pBC–3XHA–mCherry and pBC–mCherry–TgDHFR vectors and Pb mCherry parasites. We acknowledge Sanger as having made available the Materials and Lucigen as the source of the plasmid vector used to generate the PlasmoGEM resource. We thank Dr. Saman Habib (CSIR-CDRI, India), Dr. Anthony A. Holder (The Francis Crick Institute, UK), Drs. Photini Sinnis and Sean Prigge (Johns Hopkins University, USA) for anti-ICT1, anti-MSP1, anti-UIS4 and anti-ACP antibodies, respectively. We also thank Dr. Puran Singh Sijwali (CCMB, Hyderabad) for the pET32a–PfAtg8 plasmid. We acknowledge the THUNDER (BSC0102) and MOES (GAP0118) Intravital and Confocal microscopy facility of CSIR-CDRI. We thank Rima Ray Sarkar and Anil Kumar for their technical assistance with microscopy. The University Grants Commission and Council of Scientific and Industrial Research, Government of India research fellowships supported AM and AV. This manuscript is CDRI Communication No. 10683.
Abbreviations
- Atg
Autophagy-related genes
- Atg4
Autophagy-related protein 4
- Otu
Ovarion tumor unit
- EEF
Exoerythrocytic forms
- HA
Hemagglutinin
- Hsp70
Heat Shock Protein70
- MSP
Merozoite Surface Protein 1
- ACP
Acyl Carrier Protein
- UIS4
Upregulated in infectious sporozoites gene 4
- GFP
Green fluorescent protein
- TRAP
Thrombospondin-related anonymous protein
- iRBC
Infected RBC
- UTR
Untranslated region
- IV
Intravenously
- IFA
Immunofluorescence assay
- hDHFR
Human dihydrofolate reductase
Author contributions
AM and SM conceived the idea, designed and performed the experiments, analyzed the data and wrote the manuscript. AV performed the experiments. All the authors have read and approved the manuscript.
Funding
The work was supported by the CSIR-CDRI innovative idea grant [CII7045].
Availability of data and materials
All data are available within this manuscript, and raw data are available from the corresponding author upon reasonable request. Materials generated in this study are available from the corresponding author on request.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The current study does not involve human samples. All animal procedures were approved by the Institutional Animal Ethics Committee at CSIR-Central Drug Research Institute, India (IAEC/2018/3).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15:344–357. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen X, Klionsky DJ. An overview of macroautophagy in yeast. J Mol Biol. 2016;428:1681–1699. doi: 10.1016/j.jmb.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- 8.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 9.Noda NN, Inagaki F. Mechanisms of autophagy. Annu Rev Biophys. 2015;44:101–122. doi: 10.1146/annurev-biophys-060414-034248. [DOI] [PubMed] [Google Scholar]
- 10.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–3298. doi: 10.1091/mbc.e07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakatogawa H, Ichimura Y, Ohsumi Y. Atg8, a ubiquitin-like protein required for autophagosome formation mediates membrane tethering and hemifusion. Cell. 2007 doi: 10.1016/j.cell.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Fujita N, Hayashi-Nishino M, Fukumoto H, et al. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell. 2008;19:4651–4659. doi: 10.1091/mbc.e08-03-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatogawa H, Ishii J, Asai E, Ohsumi Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy. 2012;8:177–186. doi: 10.4161/auto.8.2.18373. [DOI] [PubMed] [Google Scholar]
- 15.Choy A, Dancourt J, Mugo B, et al. The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervantes S, Bunnik EM, Saraf A, et al. The multifunctional autophagy pathway in the human malaria parasite Plasmodium falciparum. Autophagy. 2014 doi: 10.4161/auto.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennand A, Gualdrón-lópez M, Coppens I, et al. Autophagy in parasitic protists: unique features and drug targets. Mol Biochem Parasitol. 2011;177:83–99. doi: 10.1016/j.molbiopara.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Duszenko M, Ginger ML, Brennand A, et al. Autophagy in protists. Autophagy. 2011;7:127–158. doi: 10.4161/auto.7.2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura K, Kishi-itakura C, Tsuboi T, et al. Autophagy-related Atg8 localizes to the apicoplast of the human malaria parasite Plasmodium falciparum. PLoS ONE. 2012 doi: 10.1371/journal.pone.0042977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor P, Jayabalasingham B, Voss C, et al. Characterization of the ATG8-conjugation system in 2 Plasmodium species with special focus on the liver stage possible linkage between the apicoplastic and autophagic systems ? Autophagy. 2014 doi: 10.4161/auto.27166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eickel N, Kaiser G, Prado M, et al. Features of autophagic cell death in Plasmodium liver-stage parasites. Autophagy. 2013;9:568–580. doi: 10.4161/auto.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlins AM, Ben-rached F, Williams RAM, et al. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013 doi: 10.4161/auto.25832. [DOI] [PubMed] [Google Scholar]
- 23.Walczak M, Ganesan SM, Niles JC, Yeh E. ATG8 is essential specifically for an autophagy-independent function in apicoplast biogenesis in blood-stage malaria parasites. mBio. 2018;9:1–13. doi: 10.1128/mBio.02021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lévêque MF, Berry L, Cipriano MJ, et al. Autophagy-related protein ATG8 has a noncanonical function for apicoplast inheritance in Toxoplasma gondii. mBio. 2015 doi: 10.1128/mBio.01446-15.Invited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker DM, Mahfooz N, Kemme KA, et al. Plasmodium falciparum erythrocytic stage parasites require the putative autophagy protein PfAtg7 for normal growth. PLoS ONE. 2013;8:2–9. doi: 10.1371/journal.pone.0067047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal P, Tripathi A, et al. Autophagy-related protein ATG18 regulates apicoplast biogenesis in apicomplexan parasites. mBio. 2017;8:5–01468. doi: 10.1128/mBio.01468-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong-hap MA, Mouammine A, Daher W, et al. Regulation of ATG8 membrane association by ATG4 in the parasitic protist Toxoplasma gondii. Autophagy. 2013 doi: 10.4161/auto.25189. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Meister TR, Walczak M, et al. A mutagenesis screen for essential plastid biogenesis genes in human malaria parasites. PLoS Biol. 2019 doi: 10.1371/journal.pbio.3000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta R, Mishra A, Choudhary HH, et al. Secreted protein with altered thrombospondin repeat (SPATR) is essential for asexual blood stages but not required for hepatocyte invasion by the malaria parasite Plasmodium berghei. Mol Microbiol. 2020;113:478–491. doi: 10.1111/mmi.14432. [DOI] [PubMed] [Google Scholar]
- 30.Janse CJ, Ramesar J, Waters AP. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc. 2006;1:346–356. doi: 10.1038/nprot.2006.53. [DOI] [PubMed] [Google Scholar]
- 31.Gomes AR, Bushell E, Schwach F, et al. A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe. 2015;17:404–413. doi: 10.1016/j.chom.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godiska R, Mead D, Dhodda V, et al. Linear plasmid vector for cloning of repetitive or unstable sequences in Escherichia coli. Nucleic Acids Res. 2010;38:e88. doi: 10.1093/nar/gkp1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charan M, Choudhary HH, Singh N, et al. [Fe-S] cluster assembly in the apicoplast and its indispensability in mosquito stages of the malaria parasite. FEBS J. 2017;284:2629–2648. doi: 10.1111/febs.14159. [DOI] [PubMed] [Google Scholar]
- 34.Al-Nihmi FMA, Kolli SK, Reddy SR, et al. A novel and conserved plasmodium sporozoite membrane protein SPELD is required for maturation of exo-erythrocytic forms. Sci Rep. 2017;7:40407. doi: 10.1038/srep40407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhary HH, Gupta R, Mishra S. PKAc is not required for the preerythrocytic stages of Plasmodium berghei. Life. 2019;2:1–11. doi: 10.26508/lsa.201900352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruña-Romero O, Hafalla JC, González-Aseguinolaza G, et al. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001;31:1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 37.Narwal SK, Nayak B, Mehra P, Mishra S. Protein kinase 9 is not required for completion of the Plasmodium berghei life cycle. Microbiol Res. 2022;260:127051. doi: 10.1016/j.micres.2022.127051. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji M, Mombaertst P, Lefrancoist LEO, et al. Gamma delta T cells contribute to immunity against the liver stages of malaria in c43 T-cell-deficient mice. Proc Natl Acad Sci USA. 1994;91:345–349. doi: 10.1073/pnas.91.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller A-K, Labaied M, Kappe SHI, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 40.Holder AA, Freeman RR. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981;294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher JR, Prigge ST. Plasmodium falciparum acyl carrier protein crystal structures in disulfide-linked and reduced states and their prevalence during blood stage growth. Proteins. 2010;78:575–588. doi: 10.1002/prot.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaishya S, Kumar V, Gupta A, et al. Polypeptide release factors and stop codon recognition in the apicoplast and mitochondrion of Plasmodium falciparum. Mol Microbiol. 2016;100:1080–1095. doi: 10.1111/mmi.13369. [DOI] [PubMed] [Google Scholar]
- 43.Jayabalasingham B, Bano N, Coppens I. Metamorphosis of the malaria parasite in the liver is associated with organelle clearance. Cell Res. 2010;20:1043–1059. doi: 10.1038/cr.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra A, Srivastava PN, H SA, Mishra S, Autophagy protein Atg7 is essential and druggable for maintaining malaria parasite cellular homeostasis and organelle biogenesis. BioRxiv. 2023 doi: 10.1101/2023.08.16.553492. [DOI] [Google Scholar]
- 45.Hirata E, Ohya Y, Suzuki K. Atg4 plays an important role in efficient expansion of autophagic isolation membranes by cleaving lipidated Atg8 in Saccharomyces cerevisiae. PLoS ONE. 2017;12:e0181047. doi: 10.1371/journal.pone.0181047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta G, Hossain ME, Asad M, et al. Plasmodium falciparum OTU-like cysteine protease (PfOTU) is essential for apicoplast homeostasis and associates with noncanonical role of Atg8. Cell Microbiol. 2017;19:1–15. doi: 10.1111/cmi.12748. [DOI] [PubMed] [Google Scholar]
- 47.Kirisako T, Ichimura Y, Okada H, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263–276. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichimura Y, Kirisako T, Takao T, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 49.Besteiro S, Brooks CF, Striepen B, Dubremetz J-F. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 2011;7:e1002416. doi: 10.1371/journal.ppat.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M, Wang C, Otto TD, et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science. 2018 doi: 10.1126/science.aap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joy S, Thirunavukkarasu L, Agrawal P, et al. Basal and starvation-induced autophagy mediates parasite survival during intraerythrocytic stages of Plasmodium falciparum. Cell Death Discov. 2018;4:43. doi: 10.1038/s41420-018-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng L, Tian Y, Wang Y, et al. Toxoplasma TgAtg8-TgAtg3 interaction primarily contributes to apicoplast inheritance and parasite growth in tachyzoite. Microbiol Spectr. 2022;10:e0149521. doi: 10.1128/spectrum.01495-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomova C, Humbel BM, Geerts WJC, et al. Membrane contact sites between apicoplast and ER in Toxoplasma gondii revealed by electron tomography. Traffic. 2009;10:1471–1480. doi: 10.1111/j.1600-0854.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 54.Tonkin CJ, Struck NS, Mullin KA, et al. Evidence for Golgi-independent transport from the early secretory pathway to the plastid in malaria parasites. Mol Microbiol. 2006;61:614–630. doi: 10.1111/j.1365-2958.2006.05244.x. [DOI] [PubMed] [Google Scholar]
- 55.Nijman SMB, Luna-Vargas MPA, Velds A, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Bushell E, Gomes AR, Sanderson T, et al. Functional profiling of a Plasmodium genome reveals an abundance of essential genes. Cell. 2017;170:260–272.e8. doi: 10.1016/j.cell.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanway RR, Bushell E, Chiappino-Pepe A, et al. Genome-scale identification of essential metabolic processes for targeting the Plasmodium liver stage. Cell. 2019;179:1112–1128.e26. doi: 10.1016/j.cell.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within this manuscript, and raw data are available from the corresponding author upon reasonable request. Materials generated in this study are available from the corresponding author on request.