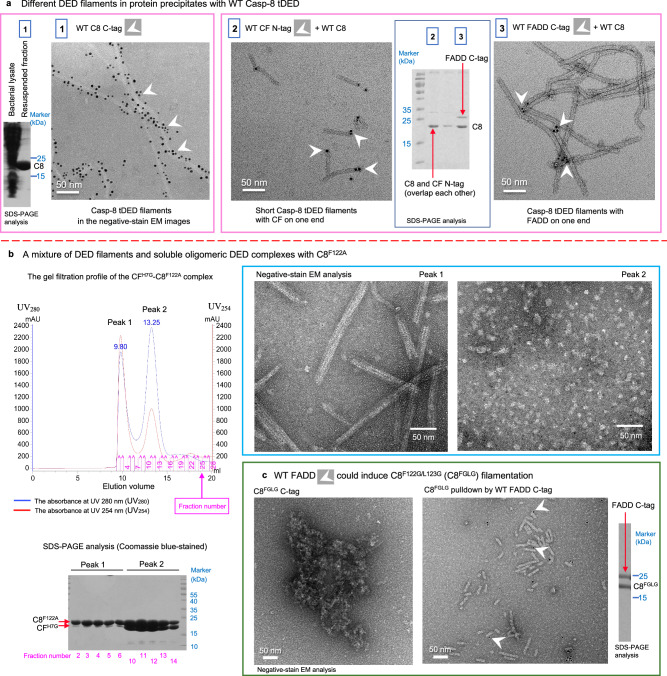
Fig. 1. Casp-8tDED mutations impair Casp-8 self-filamentation but facilitate complex reconstitution.
a The wild-type (WT) Casp-8tDED (C8) predominantly forms filaments. Negative-stain electron microscopy (EM) and Coomassie blue-stained SDS-PAGE analysis of precipitates formed by the wild-type protein or complexes and resuspended in water. White arrowheads indicate some His-tagged proteins detected by Nanogold. Sample 1: WT Casp-8tDED with a C-terminal His-tag (WT C8 C-tag). Sample 2: WT Casp-8tDED plus WT cFLIPtDED with an N-terminal His-tag (WT CF N-tag). Sample 3: WT Casp-8tDED plus WT FADD with a C-tag. b Casp-8tDED_F122A mutant aids in the reconstitution of soluble DED complexes. SEC profile of the binary CFH7G-Casp-8tDED_F122A complex and negative-stain EM and SDS-PAGE analyses of the peak fractions. AU absorbance units. c Monomeric Casp-8tDED_F122G/L123G mutant (C8FGLG) forms short filaments in the presence of WT FADD. Negative-stain EM images show that C8FGLG purified by SEC cannot form filaments, whereas it forms short filaments in the presence of WT FADD, although FADD mostly dissociated. White arrowheads indicate C-terminal His-tagged (C-tag) FADD detected by Nanogold. The SDS-PAGE analysis of the EM sample of the binary complex is also shown. All micrograph and biochemical data were repeated independently twice with similar results. Scale bar = 50 nm. Source data are provided as a Source Data file.