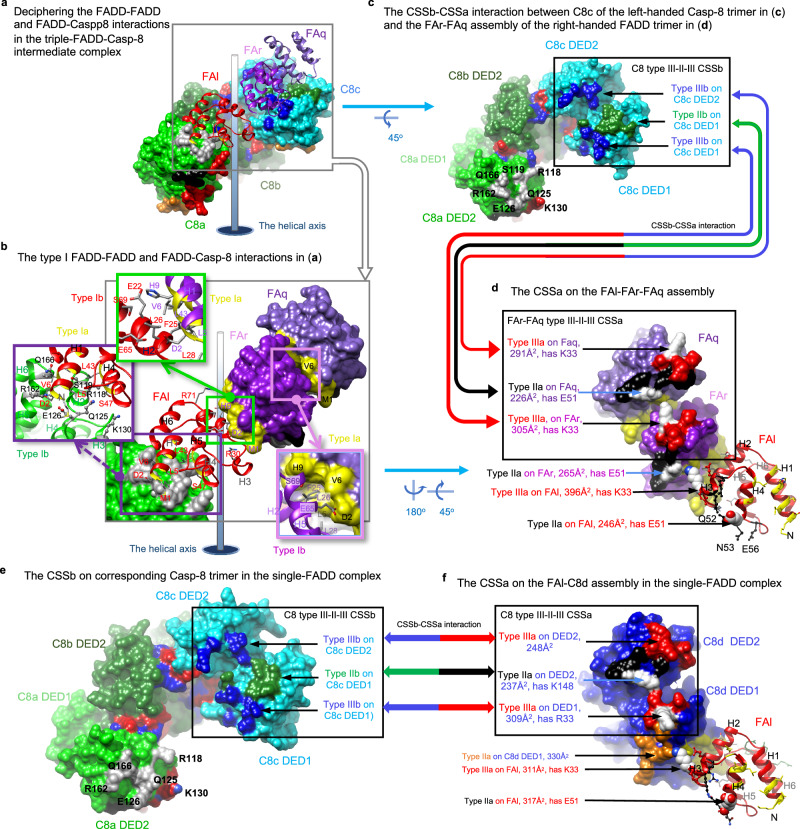
Fig. 4. FADDDED-FADDDED and FADDDED-Casp-8tDED assemblies in the intermediate complex.
a Shows that FADDDED molecules FAl, FAr, and FAq assemble a right-handed trimer that binds a left-handed Casp-8tDED trimer in the triple-FADD-Casp-8 intermediate complex. The molecules are labeled and colored as in Fig. 3a, b. FAq and FAr refer to FADDDED (FA) with the chain ID q and r, respectively. b Shows the interface residues on type I FADDDED-FADDDED and FADDDED-Casp-8tDED interfaces. The green and pink boxes show the residues on the type I interfaces in the triple-FADD, i.e., the FAl-FAr-FAq assembly, while the purple box shows the interface residues between Casp-8tDED molecule C8a and FADDDED molecule FAl. The type Ia surfaces and corresponding ribbons of FADDDED are colored in yellow. The interface residues of FADDDED molecules FAl, FAr, and FAq are labeled in red, purple, and black, respectively. while those on Casp-8tDED molecule C8a are labeled in black. c, d Black boxes and double-headed arrows highlight the CSSb-CSSa interaction between the Casp-8tDED trimer (c) and FADDDED trimer (d) of the triple-FADD complex. In d, type IIa and IIIa surfaces are colored in black and red, respectively, in which functionally important FADD residues K33 and E51 are highlighted in white. The size of each interface on FADDDED is shown in parentheses. Notably, in c, the type Ib residues of Casp-8tDED molecule C8a are labeled in black. e-f, Black boxes and double-headed arrows highlight the corresponding CSSb-CSSa interaction in the single-FADD complex, between the corresponding Casp-8tDED trimer (e) and single-FADD-single-Casp-8 assembly (f) with similar orientations to the those in c and d, respectively. Notably, in f, Casp-8 surfaces are colored as in Fig. 3a, except that functionally important Casp-8 residues R33 and K14838 have white surfaces.