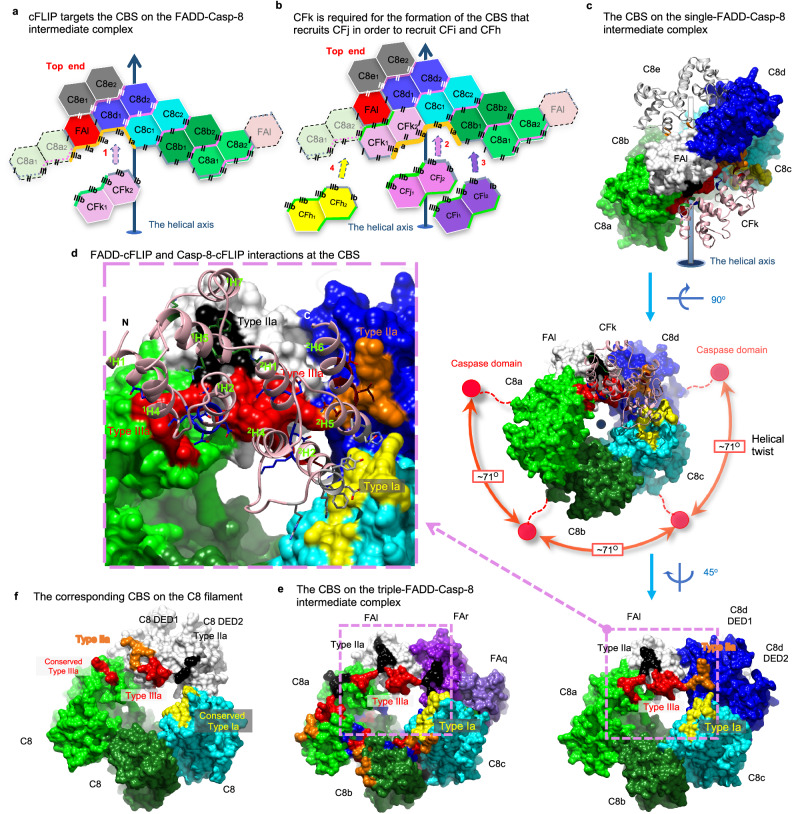
Fig. 5. cFLIP targets the CBS of the FADD-Casp-8 intermediate complex and further caps it.
a Thick orange lines highlight the CBS of the single-FADD intermediate complex shown in 2D representation. The CBS is targeted by cFLIPtDED molecule CFk, as the first step of cFLIP recruitment. b Thick orange lines highlight the CBS generated after the recruitment of cFLIPtDED molecule CFk to the single-FADD complex. The CBS could recruit another cFLIPtDED molecule CFj. This process repeats until the complex is capped by cFLIP and reaches a maximum capacity of four cFLIP molecules. c Shows FADDDED-cFLIPtDED and Casp-8tDED-cFLIPtDED assemblies in the single-FADD complex. Surface representations of the single-FADD intermediate complex viewed from different angles show that the CBS contains a type Ia surface (yellow) from Casp-8tDED molecule C8c DED1, a type IIa surface (orange) from Casp-8tDED molecule C8d DED1, a type IIa (black) and a type IIIa (red) surfaces from FADDDED molecule FAl, and a type IIIa surface (red) from Casp-8tDED molecule C8a DED2. Red balls in the middle panel indicate the possible locations of the protease domains. d The pink box highlights FADDDED-cFLIPtDED and Casp-8tDED-cFLIPtDED interactions in c. cFLIPtDED molecule CFk is shown as pink ribbons, while its interface residues are shown as sticks. In c–e, FADDDED and Casp-8tDED are labeled and colored as in Fig. 2a, except that FADDDED molecule FAl is colored in white for clarity and for the comparison with the white Casp-8tDED molecule in f. e The pink box highlights the corresponding CBS surfaces in the triple-FADD intermediate complex. Notably, two type IIa surfaces (black) are from FADDDED molecules FAl and FAr. f, The corresponding CBS surfaces in the C8 filament (5L08 [10.2210/pdb5L08/pdb])38. White surfaces highlight the C8 molecule that provides two type IIa (black and orange) and a type IIIa (red) surfaces.