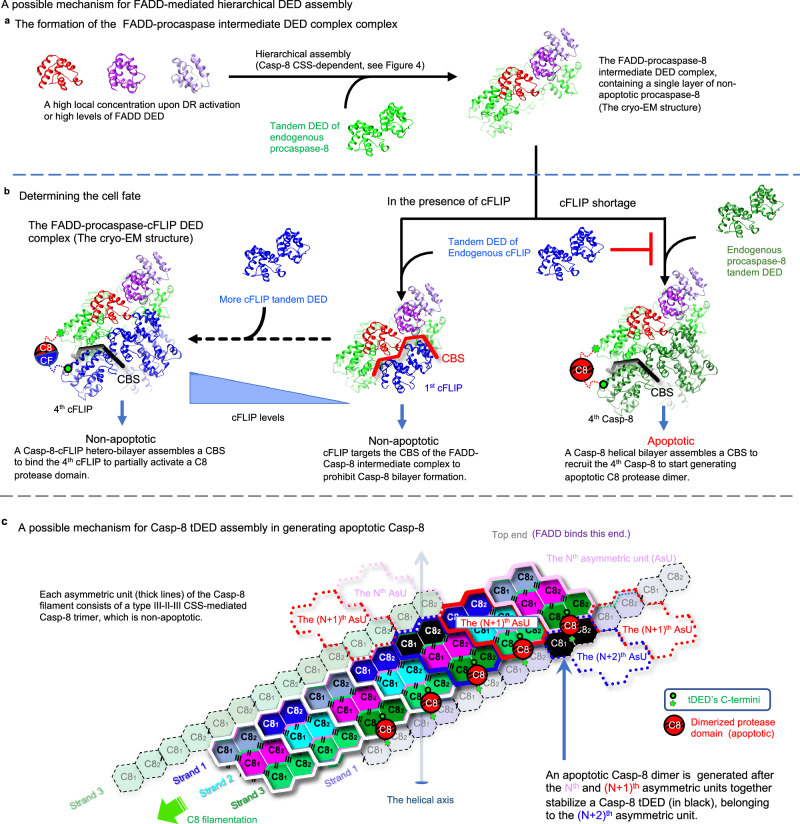
Fig. 7. DED assembly mechanisms in regulating apoptotic signaling.
a, b Propose a hierarchical FADD-mediated DED assembly mechanism for the FADD-Casp-8 and FADD-Casp-8-cFLIP complexes. a In the first stage, the formation of a FADD-Casp-8 intermediate complex requires CSS-mediated self-assembly of endogenous Casp-8 and also a high local concentration of FADD to interact with the Casp-8 assembly. Notably, the single-FADD intermediate complex assembles in a similar manner. b In the second stage, cFLIP targets the CBS on the intermediate complex, terminating hierarchical apoptotic signaling. This process generates a Casp-8-cFLIP hetero-double layer, licensing local Casp-8 activation. However, when cFLIP is depleted, Casp-8 binds the same CBS, forming Casp-8 double layers and hence filaments to execute apoptosis. This unveils structurally how the levels of cFLIP mechanically regulate apoptotic signaling. c A 2D representation illustrates tDED assembly in a Casp-8 filament, elucidating the mechanism of generating apoptotic Casp-8. The tDED is colored as per the suggested model for EMD-11939 in Supplementary Fig. 11g. Each asymmetric unit is highlighted by thick lines. Two asymmetric units, for example, the Nth and (N + 1)th, of the Casp-8 filament assemble a Casp-8 double layer and hence a CBS, stabilizing the first Casp-8tDED (black) of the next forming (N + 2)th asymmetric unit. This arrangement allows its protease domain and the protease domain of the third Casp-8tDED located in the Nth asymmetric unit to form an apoptotic dimer (depicted as red balls). See also Supplementary Fig. 11f, g for apoptotic Casp-8 formation.