Table 2. Evaluation of Functional Groups on the Catalytic Performance.
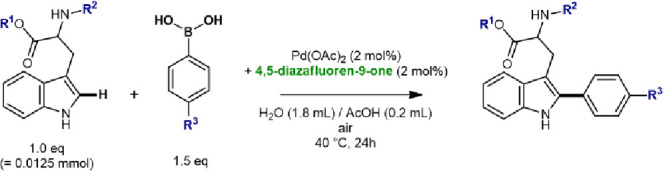
| entry | R1 | R2 | R3 | conversion (%)a |
|---|---|---|---|---|
| 1 | Me | Ac | H | 92 (62)b |
| 2c | Me | Ac | H | 73 |
| 3d | Me | Ac | H | 72 |
| 4 | Me | Ac | Me | 60 |
| 5 | Me | Ac | OMe | 83 |
| 6 | Me | Ac | F | 76 |
| 7 | Me | Ac | OH | 82 |
| 8 | Me | Ac | CH2OH | 98 |
| 9 | Me | Ac | NMe2 | 82 |
| 10 | Me | H (−NH3+) | H | <1 |
| 11 | Me | NAc-Gly- | H | 50 |
| 12 | H (−COOH) | Ac | H | 53 |
| 13 | –Ala-OMe | Ac | F | 73 |
| 14 | NAcTrpLysLeuValGlyAlaOH | F | 74 (95)e | |
Conversion of the tryptophan reactant with 2 mol % Pd and atmospheric oxygen pressure, as determined via HPLC using a calibration curve. The major product peak was identified as the arylated product via LC-MS (see the Supporting Information).
Reaction performed on a 1 mmol scale (0.0375 M) in an open flask.
Reaction with phenylboronic acid pinacol ester (1.5 equiv).
Reaction with potassium phenyltrifluoroborate (1.5 equiv).
Reaction performed with 6 mol % Pd(OAc)2/4,5-diazafluoren-9-one.
