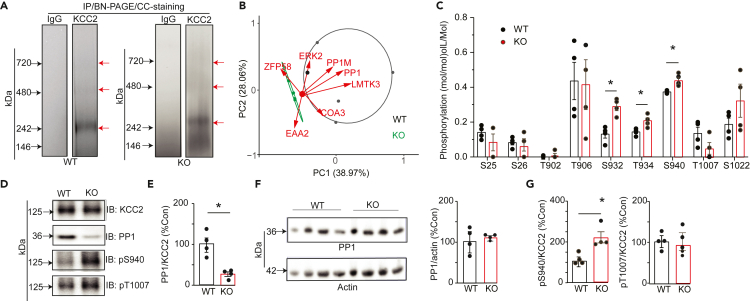
Figure 4.
Examining the effects of ablating LMTK3 on PP1 recruitment to KCC2 and its phosphorylation
(A) Solubilized brain membranes from 6- to 8-week-old WT and LMTK3-KO (KO) mice were exposed to immunopurified onKCC2 antibody or control IgG. Bound material was eluted in 2% Tween, subject to BN-PAGE, and stained with CCB. The regions of the gels indicated by the red arrows were excised, digested with trypsin, and subject to LC-MS/MS.
(B) Proteins that copurified with KCC2 from WT and KO mice were using neutral labeling, and their composition was compared using PCA for each replicate (n = 4/genotype). The proteins indicated are the principle proteins that contribute to the separation between genotypes.
(C) The phosphorylation of KCC2 was compared between genotypes using neutral labeling to determine the ratio of phosphorylated/dephosphorylated peptides for specific amino acids in the mature transporter. ∗p < 0.05; t test; n = 4 purifications.
(D) KCC2 was immunopurified from WT and KO mice. Purified material was then subject to SDS-PAGE and immunoblotted with KCC2, PP1, pS940, and pT1007 antibodies.
(E) The ratio of PP1/KCC2 immunoreactivity was determined and normalized to levels in WT; ∗p < 0.05; t test; n = 3 mice.
(F) Total brain lysates were immunoblotted with antibodies against PP1 and actin. The ratios of PP1/actin immunoreactivity were then determined and compared with WT; n = 4 mice.
(G) pS940/KCC2 and pT1007/KCC2 immunoreactivity were determined and normalized to levels in WT. ∗p < 0.05; t test; n = 3 mice. In all panels data represent mean ± SEM.