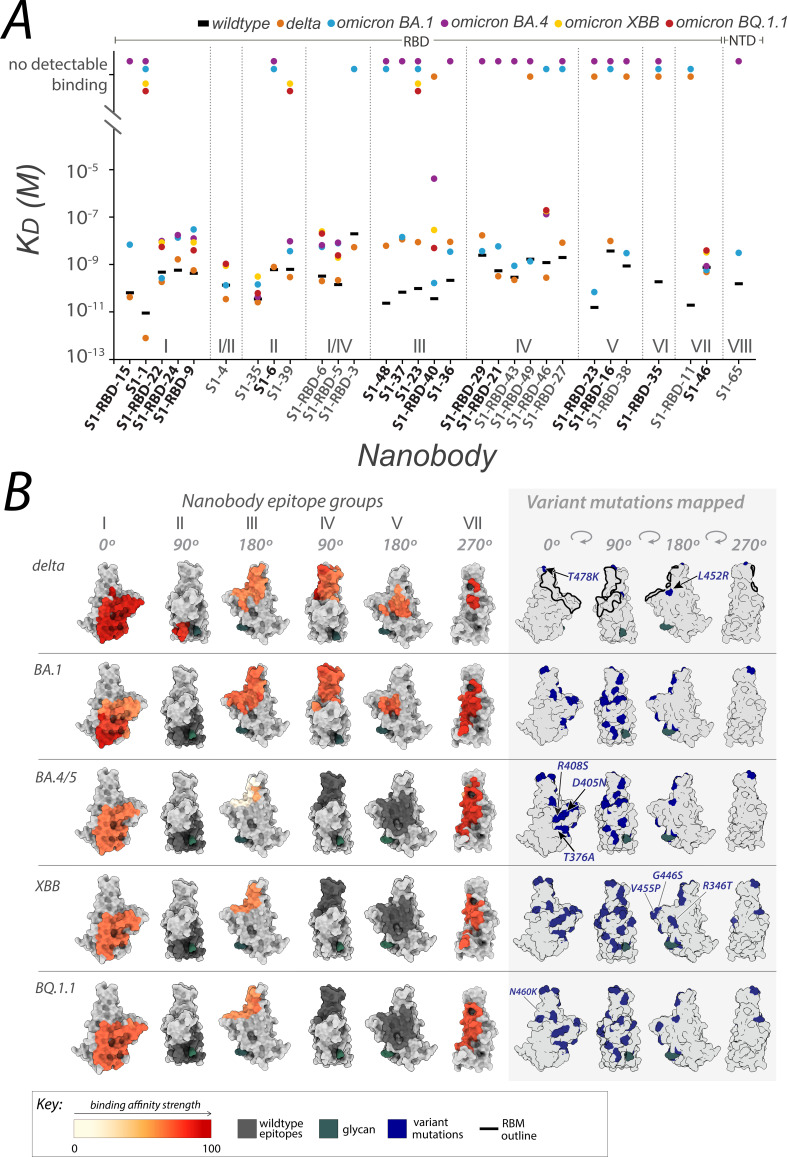
Figure 3. Affinities of the nanobody repertoire against SARS-CoV-2 variants.
(A) Each nanobody is plotted against their affinity (KD) for SARS-CoV-2 Spike S1 from wild-type, delta and omicron BA.1, BA.4, XBB, and BQ.1.1 strains. Kinetic values are summarized in . Nanobodies are characterized into their respective epitope groups as described previously (Mast et al., 2021). (B) Displayed are the structures of the receptor-binding domain (RBD) of spike delta (PDB ID: 7SBO), omicron BA.1 (PDB ID: 7T9K), omicron BA.4 RBD modeled using AlphaFold (Jumper et al., 2021), omicron XBB (PDB ID: 8IOU), and omicron BQ.1.1 (PDB ID: 8FXC). The structures feature heat-mapped epitopes of binding, ranging from pale white (weak binding to SARS-CoV-2) to dark red (strong binding to SARS-CoV-2). In the gray box, mutations specific to each variant are highlighted in blue. The nanobodies that contributed to epitope mapping are in bold in panel A. The color bar scale indicates each epitope’s binding affinity strength, represented as the normalized −log10 ratio of nanobody binding (KD) of variant versus wild-type SARS-CoV-2 Spike S1. For groups with multiple nanobodies, the average −log10 (KD) for the nanobodies within that group was calculated, then normalized to an affinity score ranging from 0 to 100 using the min and max average −log10 (KD) for that group. Higher −log10 ratios indicate stronger binding of the nanobody to the variant versus wild-type. S1-RBD-16 bound omicron BA.1 and BA.4/5 in ELISA. S1-RBD-11 was not tested against omicron BA.4. S1-65 was not tested against BA.1. Only S1-1, S1-RBD-22, S1-RBD-9, S1-4, S1-35, S1-39, S1-RBD-6, S1-RBD-5, S1-23, S1-RBD-40, S1-RBD-46, and S1-46 were tested against omicron XBB and BQ.1.1. All structure representations were generated using ChimeraX (Pettersen et al., 2021).