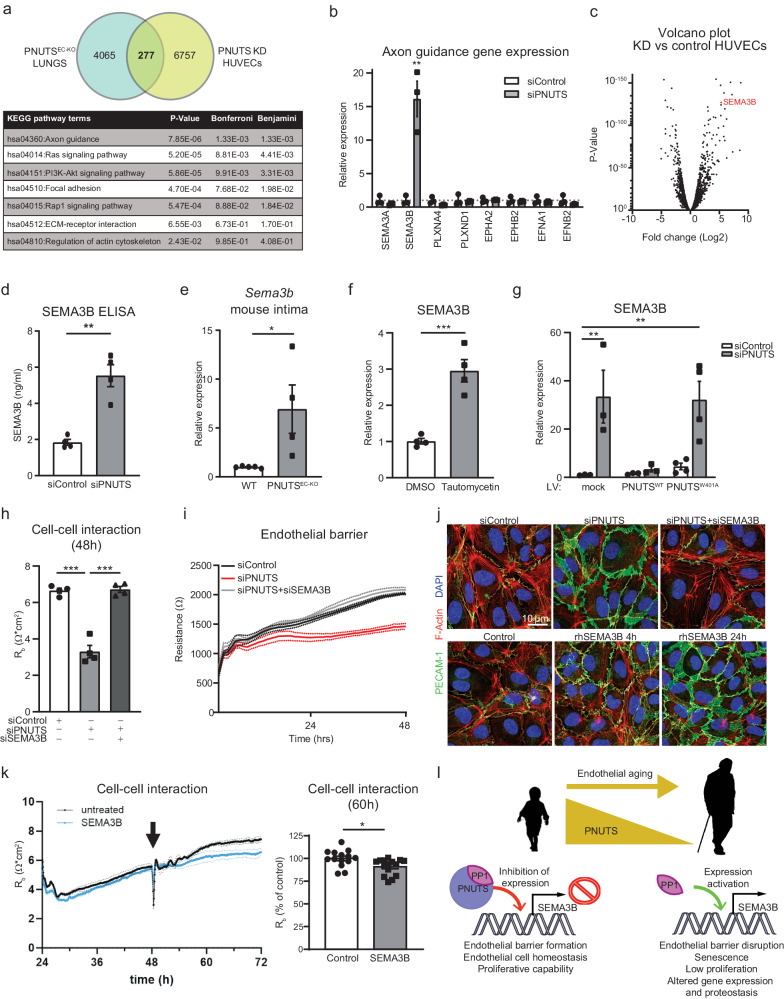
Fig. 5. PNUTS KD induces transcriptomic changes in HUVECs.
a The two subsets of RNAseq data we obtained, from endothelial-depleted PNUTS mouse lungs and PNUTS knockdown (KD) HUVECs, were compared to find common targets of PNUTS depletion. The Venn diagram depicts the finding of 277 common transcripts, which were functionally analysed using KEGG pathways analysis, shown in the table below. b Changes in axon guidance gene expression after PNUTS silencing was assessed by RT-qPCR. Expression values are relative siControl-treated HUVECs and normalized to RPSA mRNA (n = 6). c Volcano plot showing the distribution of gene expression in PNUTS KD versus control HUVECs. SEMA3B is marked in red. d Supernatant SEMA3B concentration was determined by ELISA 72 h after PNUTS silencing (n = 4). e Expression levels of Sema3b mRNA in intima samples of PNUTSEC-KO mice was assessed by RT-qPCR, relative to WT samples and normalized to Rplp0 mRNA. f HUVECs were treated with vehicle or Tautomycetin (166 nM) for 48 h and mRNA was analyzed by RT-qPCR for expression of SEMA3B, normalized to RPSA mRNA (n = 3). g Expression of SEMA3B was measured in mRNA samples of HUVECs assayed in Fig. 4j–l, relative to control cells and normalized to RPSA mRNA. h-i HUVECs were co-transfected with siPNUTS and/or siSEMA3B were subjected to ECIS for 48 h (n = 4 independent experiments, 4 biological replicates per group and experiment). h Cell-cell interaction was modeled. i Endothelial resistance was measured at 4000 Hz. j Top panel: siSEMA3B rescues the effect of PNUTS silencing on adherence junctions (shown as PECAM1 IF staining). Bottom panel: PECAM1 IF staining shows time course of change in adherens junctions upon stimulation with recombinant human SEMA3B. k Cell-cell interaction was modeled using ECIS. The arrow indicates the 48 h time point at which recombinant SEMA3B was added to the medium (or not; untreated). Quantification was performed at 60 h (n = 4 biological replicates and 3–4 technical replicates). l Graphic summary of the proposed mechanism. In young individuals, PNUTS interacts and promotes activity of PP1, which represses the expression of SEMA3B. Endothelial cells are in homeostasis and maintain their barrier function. During aging, PNUTS is repressed in endothelial cells. The absence of PNUTS inhibits PP1 function at the SEMA3B promoter activating SEMA3B expression. SEMA3B exerts repulsive signals between endothelial cells, promoting intercellular gaps and disrupting the barrier. This provokes a series of critical changes in the cells leading to cellular senescence. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars depict the standard error of the mean (SEM).