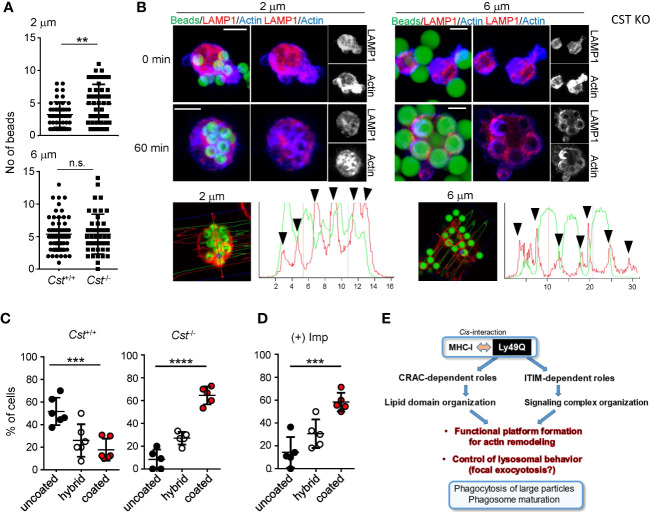
Figure 7.
Significance of sulfatide in phagocytosis. PEMϕs were prepared from Cst +/+ and Cst -/- mice, and phagocytosis of IgG-opsonized beads with different diameters was examined. (A) The numbers of ingested beads in single cells were counted. Each dot represents the number of beads ingested by a single cell. More than 30 cells were analyzed. (B) F-actin (blue) and endolysosomes (red) in PEMϕs that had ingested fluorescence-labeled beads (green) were visualized by staining with phalloidin and anti-LAMP1 antibody at the indicated time points. Histograms at the bottom show the fluorescence intensity profiles of beads (green) and F-actin (red) along the dashed lines in the left photographs. (C, D) Frequencies of cells possessing phagosomes with and without an actin coat in Cst +/+ and Cst -/- PEMϕs (C) and in Cst +/+ PEMϕs treated with imipramine (10 µM) (D). Hybrid represents cells possessing both actin-coated and actin-uncoated phagosomes. All results shown are representative of at least two independent experiments. (E) Roles of Ly49Q in phagocytosis mediated by two different functional domains. Ly49Q has a CRAC-like motif and an ITIM; the former is needed for preparing functional membrane domains via its sulfatide binding ability, and the latter recruits a panel of signaling molecules to regulate actin remodeling and the distribution of Ly49Q together with the functional membrane domain. Regulation of both protein-lipid interactions by the CRAC motif and protein−protein interactions by the ITIM is needed for phagocytosis. Statistical analyses were conducted using Mann–Whitney U test (A) and one-way ANOVA (C, D). ****P<0.0001; ***P<0.001; **P<0.01; ns, not significant. All results shown are representative of at least two separate experiments.