Abstract
Background
Retifanlimab is a humanized, hinge-stabilized immunoglobulin G4κ monoclonal antibody against human programmed cell death protein 1 (PD-1). This first-in-human, phase I study assessed the safety and efficacy of retifanlimab in patients with advanced solid tumors and identified optimal dosing.
Patients and methods
POD1UM-101 was conducted in two parts: (i) dose escalation—evaluated retifanlimab [1 mg/kg every 2 weeks (q2w), 3 or 10 mg/kg q2w or every 4 weeks (q4w)] in patients with relapsed/refractory, unresectable, locally advanced or metastatic solid tumors; (ii) cohort expansion—biomarker-unselected tumor-specific cohorts [endometrial, cervical, sarcoma, non-small-cell lung cancer (NSCLC)] received retifanlimab 3 mg/kg q2w, and tumor-agnostic cohorts received flat dosing [375 mg every 3 weeks (q3w), or 500 and 750 mg q4w]. Primary objectives were safety and tolerability; secondary objective was efficacy in selected tumor types.
Results
Thirty-seven patients were enrolled in dose escalation, 134 in PD-1 therapy-naïve tumor-specific cohort expansion (endometrial, n = 29; cervical, NSCLC, soft tissue sarcoma, each n = 35), and 45 in flat dosing (375 mg q3w, 500 and 750 mg q4w, each n = 15). No dose-limiting toxicities occurred during dose escalation; maximum tolerated dose was not reached and 3-mg/kg q2w expansion dose was selected based on safety and pharmacokinetic data. Immune-related adverse events were experienced by 40 patients (30%) in tumor-specific cohorts (most frequently hypothyroidism, hyperthyroidism, colitis, nephritis) and 6 (13%) in flat dosing (most frequently hypothyroidism, hyperthyroidism). Objective response rate (95% confidence interval) was 14% (4.8 to 30.3), 14% (3.9 to 31.7), 20% (8.4 to 36.9), and 3% (0.1 to 14.9) in advanced NSCLC, endometrial, cervical, and sarcoma tumor-specific cohorts that progressed after multiple prior systemic therapies.
Conclusions
Retifanlimab demonstrated clinical pharmacology, safety, and antitumor activity consistent with the programmed death (ligand)-1 inhibitor class. POD1UM-101 results support further exploration of retifanlimab as monotherapy and backbone immunotherapy in combination treatments, with recommended doses of 500 mg q4w and 375 mg q3w.
Key words: checkpoint inhibitor, first-in-human, PD-1 inhibitor, retifanlimab, solid tumor
Highlights
-
•
Retifanlimab demonstrated antitumor activity in this first-in-human study in patients with advanced solid tumors.
-
•
Adverse events with retifanlimab therapy were generally mild to moderate in severity and manageable with standard of care.
-
•
Antitumor activity and tolerability of retifanlimab were representative of the programmed death (ligand)-1 inhibitor class.
-
•
Flat doses of 500 mg q4w or 375 mg q3w were selected for further clinical development.
-
•
Retifanlimab provides a new therapeutic option as monotherapy or backbone immunotherapy in combination treatments.
Introduction
Immune checkpoints on tumor-specific T cells down-modulate T-cell activation, impair antitumor immunity, and impede killing of tumor cells.1,2 Following the introduction of the first successful immune checkpoint inhibitor (CPI) for patients with cancer,3 immunotherapy has become a major area of focus. CPIs targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed death (ligand)-1 [PD-(L)1], and lymphocyte-activation gene 3 (LAG-3) have been developed and are currently used alone or in combination with chemotherapy for first-line or second-line treatment of ∼50 tumor types.4, 5, 6 Combination therapy with CPIs has been investigated in various solid tumor types including combination with PD-(L)1/CTLA-4 and programmed cell death protein 1 (PD-1)/LAG-3.7, 8, 9
Retifanlimab is a humanized, hinge-stabilized immunoglobulin G4κ monoclonal antibody directed against human PD-1 to prevent interaction between PD-1 and its ligands, PD-L1 and PD-L2.10,11 Preclinical characterization shows that retifanlimab blocks PD-1/PD-L1 and PD-1/PD-L2 interactions, interrupts PD-1 signaling, and enhances antigen-induced interferon-γ release with potency comparable to replicas of nivolumab or pembrolizumab.10 Full receptor occupancy at doses ≥10 mg/kg in cynomolgus monkeys also has been demonstrated.10
The first-in-human POD1UM-101 study was designed to assess the safety and clinical activity of retifanlimab in advanced solid tumors and to establish optimal dosing for future studies. Interim results describing safety and tolerability, pharmacokinetics, pharmacodynamics, immunogenicity, and preliminary antitumor activity have been presented previously and show that retifanlimab is fully representative of the PD-1 inhibitor class.11, 12, 13 Here we report the final results of dose-finding in POD1UM-101, along with clinical experience in the initial expansion cohorts of biomarker-unselected endometrial cancer, cervical cancer, non-small-cell lung cancer (NSCLC), and soft tissue sarcoma.
Patients and methods
Study design
POD1UM-101 (NCT03059823), a first-in-human, open-label, phase I study, evaluated the safety, pharmacokinetics, pharmacodynamics, immunogenicity, and preliminary antitumor activity of retifanlimab in patients with advanced solid tumors (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102254). The study was conducted following the International Council for Harmonisation Guidelines for Good Clinical Practice and all other local applicable regulations. The study protocol was approved by institutional review boards or independent ethics committees, and patients provided written consent before enrollment.
The study was conducted in two parts: dose escalation and cohort expansion. Dose escalation enrolled patients with any relapsed/refractory, unresectable, locally advanced or metastatic solid tumor. Dose escalation followed a conventional 3 + 3 design that evaluated retifanlimab dose levels of 1 mg/kg [starting dose, every 2 weeks (q2w)], 3 mg/kg [q2w and every 4 weeks (q4w)], and 10 mg/kg (q2w and q4w; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102254) to determine the maximum tolerated dose (MTD). Where possible depending on enrollment slot availability, patients were assigned to q2w and q4w cohorts in an alternating fashion. If no MTD was defined for retifanlimab after escalation to the maximum protocol-specified dose, that dose level was designated as the maximum administered dose (MAD).
The second part of the study, cohort expansion, was designed to investigate the selected dose from dose escalation, based on safety, pharmacokinetic, and pharmacodynamic data. This part of the study included PD-1 therapy-naïve and biomarker-unselected tumor-specific cohorts (endometrial cancer, cervical cancer, sarcoma, NSCLC; up to 35 patients each). The tumor-agnostic cohorts were designed to investigate flat doses of 375 mg every 3 weeks (q3w), 500 mg q4w, and 750 mg q4w (up to 15 patients each) (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102254).
Retifanlimab was administered as an intravenous infusion over 60 min. One cycle was defined as 28 days or 4 weeks for patients receiving q2w or q4w doses, and 21 days or 3 weeks for patients receiving q3w doses. Treatment with retifanlimab continued for ≤2 years or until disease progression, alternative cancer therapy initiation, unacceptable toxicity, withdrawal of consent, or other reason for drug discontinuation. Patients who achieved confirmed complete response by immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) could discontinue after two additional cycles of treatment or continue treatment for up to 2 years based on the investigator’s judgment. No dose reduction was applicable to retifanlimab, but dose interruption up to 12 weeks was permitted to allow for toxicity recovery. After the last dose of study drug, all patients were followed for safety assessments (30 days), adverse events (AEs; 90 days), and survival assessments (every 6 months for 2 years).
Patients
Patients enrolled in the study were at least 18 years of age with histologically confirmed, unresectable, locally advanced or metastatic solid tumors for whom there was no available approved therapy with demonstrated clinical benefit or were intolerant to or declined standard therapy. Patients must also have had measurable disease as per RECIST v1.1, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, adequate organ function and bone marrow reserve, and tumor specimen collected for retrospective central PD-L1 expression testing. For all expansion cohorts, disease progression during or after one to five prior anticancer systemic treatments was required; additional disease-specific inclusions were required for the NSCLC and sarcoma cohorts (Supplementary Methods, available at https://doi.org/10.1016/j.esmoop.2024.102254).
Exclusion criteria included prior treatment with a CPI (anti-PD-1, anti-PD-L1, anti-CTLA-4) for dose expansion; grade ≥3 colitis, nephritis, ocular or neurologic toxicity or meeting Hy’s law criteria or unresolved CPI-related toxicities to grade ≤1 or baseline for dose escalation; systemic corticosteroids (≥10 mg per day prednisone or equivalent) or immunosuppressant drugs within 14 days; anticancer therapy or live virus-based vaccination within 4 weeks; or radiation therapy within 2 weeks of study treatment.
Study endpoints and assessments
The primary objective was to characterize the safety, tolerability, dose-limiting toxicities (DLTs), and MTD or MAD (if no MTD was reached) of retifanlimab, and to establish recommended phase II dose(s) for further study. Secondary objectives included investigating antitumor activity by objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Pharmacokinetics and immunogenicity [antidrug antibody (ADA)] characterization of retifanlimab for a variety of dosage schedules, pharmacodynamics, and exploratory investigation of T-cell activation in peripheral blood and tumor biopsies were additional objectives of the study.
Safety and tolerability were evaluated based on AEs as per the Common Terminology Criteria for Adverse Events version 4.03. The DLTs were defined separately for hematologic and nonhematologic events (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102254). The AEs of special interest (AESI) included grade ≥3 infusion-related reactions or cytokine release syndrome, grade ≥2 immune-related adverse events (irAEs) as per the investigator’s assessment, and abnormal liver enzymes that met the criteria for potential Hy’s law.
Tumor response defined according to RECIST v1.1 was assessed by computed tomography or magnetic resonance imaging carried out every 8 weeks for the first 24 weeks (with q2w or q4w doses), or every 9 weeks for the first 27 weeks (with q3w doses), and then every 12 weeks thereafter until death, withdrawal of consent, initiation of alternative anticancer therapy, end of study, or patient lost to follow-up. Treatment following progression was allowed as per irRECIST.
Pharmacokinetic and pharmacodynamic [anti-retifanlimab antibodies, immunohistochemistry (IHC), inflamed gene signature, T-cell proliferation] assessments are described in Supplementary Methods, available at https://doi.org/10.1016/j.esmoop.2024.102254.
Statistical analysis
Patient disposition, demographics, and baseline characteristics were summarized using descriptive statistics. AEs, including treatment-emergent adverse events (TEAEs), treatment-related adverse events (TRAEs), TEAE or TRAE severity grade ≥3, serious AEs, treatment-related serious AEs, irAEs, AESI, and infusion-related reactions, were summarized and tabulated. Pharmacokinetic and pharmacodynamic analyses were described by summary statistics.
Objective responses were categorized using RECIST v1.1 [complete response, partial response (PR), progressive disease, and stable disease] and irRECIST. A two-sided 95% exact binomial confidence interval (CI) was calculated for the ORR for each expansion cohort. DOR, PFS, and OS curves; the median DOR, PFS, and OS times; and PFS and OS rates at 3, 6, 9, and 12 months were estimated by Kaplan–Meier methods. The 95% CIs for median PFS, DOR, and OS times were calculated using the Brookmeyer and Crowley method. The 95% CIs for PFS rates at 3 and 6 months, and OS rates at 1 and 2 years, were determined by normal approximation after log(-log) transformation. The sample size of the study (up to 35 patients in each of the tumor-specific cohorts and up to 15 patients in each of the tumor-agnostic cohorts) was selected to provide meaningful assessment of safety at each of the selected doses based on the probability of seeing immune-related events at the expected underlying rate of 5%.
The dose proportionality of retifanlimab serum exposure was evaluated by using a power function regression and analysis of variance. Maximum drug concentration (Cmax) and area under the curve (AUC) were evaluated using a power model; for example, AUC = α·(doseβ) or, equivalently, log(AUC) = log(α) + β·log(dose), where linear dose proportionality is accepted if β is not significantly different from 1. All descriptive analyses were carried out using Phoenix WinNonlin v8.3.4 (Certara, Princeton, NJ), and dose-proportionality analyses were carried out using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics and disposition
As of 7 April 2020, the data cut-off date, 37 patients were enrolled in the dose-escalation cohort and 134 in PD-1 therapy-naïve tumor-specific expansion cohorts (endometrial cancer, n = 29; cervical cancer, NSCLC, soft tissue sarcoma, each n = 35; patients’ tumor types are specified in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102254). An additional 45 patients (375 mg q3w, 500 mg and 750 mg q4w, each n = 15) were enrolled in the flat-dosing tumor-agnostic expansion cohorts. A summary of patient and disease characteristics is provided for each cohort in Table 1. Median (range) patient age was 63 (32-85) years in the dose-escalation cohort, 60 (18-86) years in the tumor-specific expansion cohorts, and 63 (31-75), 60 (36-76), and 56 (30-82) years in the 375-mg q3w, 500-mg q4w, and 750-mg q4w flat-dose cohorts. Female sex and ECOG performance status of 1 were 65% and 84% in the dose-escalation cohort, 69% and 69% in the tumor-specific expansion cohorts, and 60% and 71% in the flat-dose cohorts, respectively.
Table 1.
Baseline demographics and disease characteristics (safety-assessable population)
| Variable | Retifanlimab dose-escalation cohort (n = 37) | Tumor-specific cohorts (3 mg/kg q2w retifanlimab) |
Flat-dose retifanlimab cohorts |
|||||
|---|---|---|---|---|---|---|---|---|
| NSCLC (n = 35) | Endometrial cancer (n = 29) | Cervical cancer (n = 35) | Soft tissue sarcoma (n = 35) | 375 mg q3w (n = 15) | 500 mg q4w (n = 15) | 750 mg q4w (n = 15) | ||
| Age, median (range), years | 63 (32-85) | 63 (37-75) | 64 (46-84) | 51 (29-81) | 44 (18-86) | 63 (31-75) | 60 (36-76) | 56 (30-82) |
| Sex, n (%) | ||||||||
| Female | 24 (65) | 12 (34) | 29 (100) | 35 (100) | 16 (46) | 10 (67) | 9 (60) | 8 (53) |
| Male | 13 (35) | 23 (66) | 0 | 0 | 19 (54) | 5 (33) | 6 (40) | 7 (47) |
| Race, n (%) | ||||||||
| White | 29 (78) | 34 (97) | 23 (79) | 31 (89) | 30 (86) | 9 (60) | 11 (73) | 12 (80) |
| Other | 8 (22) | 1 (3) | 6 (21) | 4 (11) | 5 (14) | 6 (40) | 4 (27) | 3 (20) |
| ECOG PS, n (%) | ||||||||
| 0 | 5 (14) | 1 (3) | 7 (24) | 17 (49) | 15 (43) | 1 (7) | 7 (47) | 5 (33) |
| 1 | 31 (84) | 34 (97) | 22 (76) | 17 (49) | 20 (57) | 14 (93) | 8 (53) | 10 (67) |
| 2 | 1 (3) | 0 | 0 | 1 (3) | 0 | 0 | 0 | 0 |
| MSI status,an (%) | ||||||||
| MSI-H | 0 | NA | 3 (10) | NA | NA | NA | NA | 0 |
| MSS | 3 (50) | NA | 19 (66) | NA | NA | NA | NA | 1 (100) |
| Unknown | 3 (50) | NA | 7 (24) | NA | NA | NA | NA | 0 |
| PD-L1 expression,bn (%) | ||||||||
| TPS ≥1% | — | 8 (23) | — | — | — | — | — | — |
| TPS <1% | — | 19 (54) | — | — | — | — | — | — |
| Unknown | — | 8 (23) | — | — | — | — | — | — |
| PD-L1 expression,bn (%) | ||||||||
| CPS ≥1% | 26 (70) | — | 13 (45) | 17 (49) | 7 (20) | 4 (27) | 7 (47) | 2 (13) |
| CPS <1% | 6 (16) | — | 11 (38) | 12 (34) | 27 (77) | 8 (53) | 5 (33) | 9 (60) |
| Unknown | 5 (14) | — | 5 (17) | 6 (17) | 1 (3) | 3 (20) | 3 (20) | 4 (27) |
| Prior systemic therapy, n (%) | ||||||||
| Chemotherapy | 35 (95) | 35 (100) | 29 (100) | 35 (100) | 33 (94) | 15 (100) | 15 (100) | 13 (87) |
| Platinum-containing | 26 (70) | 35 (100) | 28 (97) | 35 (100) | 4 (11) | 10 (67) | 11 (73) | 11 (73) |
| Adjuvant | 17 (46) | 3 (9) | 10 (34) | 11 (31) | 6 (17) | 5 (33) | 4 (27) | 3 (20) |
| Neoadjuvant | 6 (16) | 2 (6) | 3 (10) | 2 (6) | 7 (20) | 4 (27) | 4 (27) | 0 |
| First-line therapy | 9 (24) | 18 (51) | 7 (24) | 15 (43) | 14 (40) | 4 (27) | 1 (7) | 2 (13) |
| Second-line therapy | 6 (16) | 11 (31) | 9 (31) | 8 (23) | 5 (14) | 3 (20) | 1 (7) | 4 (27) |
| Third- to fifth-line therapy | 9 (24) | 5 (14) | 10 (34) | 7 (20) | 12 (34) | 5 (33) | 6 (40) | 7 (47) |
| Otherc | 7 (19) | 1 (3) | 2 (7) | 6 (17) | 1 (3) | 2 (13) | 5 (33) | 2 (13) |
| Immunotherapy | 8 (22) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Targeted therapyd | 12 (32) | 4 (11) | 3 (10) | 6 (17) | 3 (9) | 9 (60) | 5 (33) | 5 (33) |
| Prior radiotherapy, n (%) | 20 (54) | 11 (31) | 19 (66) | 32 (91) | 24 (69) | 9 (60) | 8 (53) | 9 (60) |
| Prior surgery, n (%) | 37 (100) | 20 (57) | 28 (97) | 21 (60) | 32 (91) | 15 (100) | 14 (93) | 11 (73) |
CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; MSI-H, microsatellite instability-high; MSS, microsatellite stable; NA, not applicable; NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; TPS, tumor proportion score.
Pretreatment endometrial cancer tumor biopsies (archival, fresh, or a combination) were tested for MSI status by local laboratory, and then confirmed by central laboratory. The total number of patients with endometrial cancer was 6 in the dose-escalation cohort, 29 in the endometrial cancer tumor-specific cohort, and 1 in the 750-mg q4w flat-dose cohort.
Pretreatment tumor biopsies (archival, fresh, or a combination) were tested by central laboratory.
As reported by the investigator.
Targeted therapy included bevacizumab, cabozantinib, everolimus, exemestane, olaparib, pazopanib, PF-06647020, ramucirumab, regorafenib, vemurafenib (dose-escalation cohort); afatinib, bevacizumab, BI695502, osimertinib (NSCLC cohort); bevacizumab, temsirolimus (endometrial cancer cohort); everolimus, tisotumab vedotin (cervical cancer cohort); palbociclib, pazopanib (soft tissue sarcoma cohort); AR42, bevacizumab, cediranib, cobimetinib, everolimus, HMPL-453, MEK162, neratinib, olaparib, panitumumab, pazopanib, pertuzumab, regorafenib, sorafenib, trastuzumab, vemurafenib (flat-dose cohorts).
In dose escalation, eight patients had previously received CPI therapy. Greater than two lines of prior therapy were received by 24% of patients in the dose-escalation cohort, 25% in tumor-specific expansion cohorts, and 40% in flat-dose cohorts. The proportion of patients who had prior platinum-containing therapy was 70% in the dose-escalation cohort, 100% for both NSCLC and cervical cancer, 97% for endometrial cancer, and 11% for sarcoma tumor-specific dose-escalation cohorts, and 67% for 375-mg q3w cohort, and 73% for both 500-mg and 750-mg q4w flat-dose cohorts (Table 1).
At data cut-off, two patients (5%) had completed 2-year treatment in the dose-escalation cohort and 35 patients (95%) had discontinued treatment due to disease progression; no patient discontinued study treatment due to TEAEs. In the tumor-specific expansion cohorts (n = 134), 118 patients (88%) had discontinued treatment due to disease progression [92 patients (69%)] and TEAEs {19 patients (14%); colitis [4 (3%)], cardiac failure, cardiovascular insufficiency, myocardial infarction, myocarditis, diarrhea, general physical health deterioration, peripheral edema, increased transaminases, failure to thrive, type 1 diabetes mellitus, brain edema, nephritis, female genital tract fistula, pneumothorax, pulmonary embolism [each 1 (0.7%)]}, eight patients (6%) had completed 2-year treatment, and eight patients (6%) were having ongoing retifanlimab treatment. In the flat-dose cohorts (n = 45), 44 patients (98%) had discontinued treatment due to disease progression [40 patients (89%)] and TEAEs {three patients (7%); tumor pain [1 (2%); 375 mg q3w], iritis [1 (2%); 500 mg q4w], pneumonitis [1 (2%); 750 mg q4w]} and one patient (2%) had completed 2-year study treatment.
Safety and tolerability
In the dose-escalation cohorts, patients received a median (range) of 4 (1-25) infusions of retifanlimab; the median (range) duration of treatment was 1.4 (0.03-22.9) months. Retifanlimab was well tolerated over a dose range of 1-10 mg/kg q2w; no DLTs were reported at any dose level during dose escalation. The MAD was 10 mg/kg q2w, with no MTD exceeded or defined. TRAEs were reported by 26 patients (70%) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102254). Four patients (11%) experienced five events of grade 3 or 4 TRAEs, all of which occurred at the 3-mg/kg q2w dose [increased lipase (two patients, grade 3; one patient, grade 4), vulvovaginal inflammation and anal inflammation (one patient, grade 3)]. A single serious TRAE of aphasia was reported in a patient with new brain metastases. irAEs experienced in the dose-escalation cohorts included hypothyroidism [4 (11%)], hyperthyroidism [2 (5%)], and thyroiditis and rash [one patient each (3%)].
The 3-mg/kg q2w dose was selected for cohort expansion based on safety, pharmacokinetic, and pharmacodynamic data (see Pharmacokinetics and immunogenicity, and Pharmacodynamics and exploratory translational analyses sections). Patients received a median (range) of 7 (1-52) infusions of retifanlimab 3 mg/kg q2w in the tumor-specific expansion cohorts; the median (range) duration of treatment was 2.8 (0.03-24.9) months. Among 134 patients in the tumor-specific expansion cohorts, TEAEs were reported by 125 patients (93%); the most common TEAEs were fatigue [23 (17%)], diarrhea [21 (16%)], nausea [17 (13%)], and anemia [16 (12%)] (Table 2). TRAEs were reported by 76 patients (57%) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102254); 42 patients (31%) experienced a serious TEAE. Treatment-emergent AESIs were reported by 49 patients (37%) in the tumor-specific expansion cohorts, including grade 3 or 4 AESI as per investigator in 12 patients (9%) consisting of colitis (grade 3) in three patients, both amylase (grade 3) and lipase increased (grade 3 and grade 4) in one patient, and amylase increased (grade 3), hypothyroidism (grade 3), maculopapular rash (grade 3), nephritis (grade 3), pneumonitis (grade 3), transaminases increased (grade 3), and type 1 diabetes mellitus (grade 3) in one patient each; an additional one patient had a grade 3 infusion-related reaction.
Table 2.
Most common any-grade TEAEs occurring in ≥10% of patients in any retifanlimab dosing cohort, and corresponding grade ≥3 TEAEs (safety-assessable population)
| Retifanlimab dosing |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose escalation (n = 37) |
3 mg/kg q2w (n = 134) |
375 mg q3w (n = 15) |
500 mg q4w (n = 15) |
750 mg q4w (n = 15) |
||||||
| MedDRA preferred term, n (%) | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 |
| Any TEAE | 37 (100) | 27 (73) | 125 (93) | 63 (47) | 14 (93) | 7 (47) | 15 (100) | 5 (33) | 14 (93) | 7 (47) |
| Fatigue | 16 (43) | 0 | 23 (17) | 1 (1) | 2 (13) | 0 | 5 (33) | 0 | 3 (20) | 0 |
| Anemia | 7 (19) | 6 (16) | 16 (12) | 3 (2) | 2 (13) | 1 (7) | 4 (27) | 1 (7) | 6 (40) | 3 (20) |
| Diarrhea | 3 (8) | 0 | 21 (16) | 1 (1) | 1 (7) | 0 | 2 (13) | 0 | 1 (7) | 0 |
| Nausea | 8 (22) | 0 | 17 (13) | 0 | 1 (7) | 0 | 1 (7) | 0 | 4 (27) | 0 |
| Abdominal pain | 1 (3) | 0 | 15 (11) | 1 (1) | 1 (7) | 1 (7) | 0 | 0 | 1 (7) | 0 |
| Constipation | 1 (3) | 0 | 15 (11) | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 |
| Hypothyroidism | 4 (11) | 0 | 13 (10) | 1 (1) | 0 | 0 | 1 (7) | 0 | 3 (20) | 0 |
| Vomiting | 7 (19) | 0 | 13 (10) | 0 | 0 | 0 | 1 (7) | 0 | 4 (27) | 0 |
| Pyrexia | 8 (22) | 0 | 12 (9) | 1 (1) | 0 | 0 | 2 (13) | 0 | 1 (7) | 0 |
| Dehydration | 7 (19) | 1 (3) | 3 (2) | 0 | 1 (7) | 0 | 1 (7) | 0 | 0 | 0 |
| Blood ALP increased | 6 (16) | 2 (5) | 3 (2) | 2 (1.5) | 3 (20) | 1 (7) | 4 (27) | 2 (13) | 2 (13) | 0 |
| Cough | 6 (16) | 0 | 10 (7.5) | 0 | 2 (13) | 0 | 1 (7) | 0 | 3 (20) | 0 |
| Decreased appetite | 6 (16) | 0 | 10 (7.5) | 0 | 1 (7) | 0 | 2 (13) | 0 | 0 | 0 |
| Arthralgia | 4 (11) | 0 | 5 (4) | 0 | 0 | 0 | 1 (7) | 0 | 3 (20) | 0 |
| Hypertension | 4 (11) | 2 (5) | 3 (2) | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus | 4 (11) | 0 | 9 (7) | 0 | 2 (13) | 0 | 1 (7) | 0 | 2 (13) | 0 |
| Tumor flare | 4 (11) | 0 | 1 (1) | 0 | 2 (13) | 0 | 0 | 0 | 1 (7) | 0 |
| Tumor pain | 4 (11) | 1 (3) | 0 | 0 | 3 (20) | 2 (13) | 2 (13) | 0 | 1 (7) | 0 |
| Urinary tract infection | 4 (11) | 1 (3) | 11 (8) | 3 (2) | 1 (7) | 0 | 0 | 0 | 0 | 0 |
| ALT increased | 0 | 0 | 7 (5) | 1 (1) | 2 (13) | 0 | 0 | 0 | 2 (13) | 0 |
| Amylase increased | 0 | 0 | 3 (2) | 3 (2) | 2 (13) | 2 (13) | 0 | 0 | 0 | 0 |
| AST increased | 2 (5) | 1 (3) | 8 (6) | 3 (2) | 3 (20) | 0 | 1 (7) | 0 | 2 (13) | 0 |
| Blood bilirubin increased | 3 (8) | 2 (5) | 4 (3) | 0 | 2 (13) | 0 | 1 (7) | 1 (7) | 1 (7) | 1 (7) |
| Dyspnea | 1 (3) | 0 | 13 (10) | 3 (2) | 2 (13) | 1 (7) | 0 | 0 | 0 | 0 |
| Gastritis | 1 (3) | 0 | 2 (1.5) | 0 | 2 (13) | 0 | 1 (7) | 0 | 0 | 0 |
| Hypertriglyceridemia | 0 | 0 | 2 (1.5) | 1 (1) | 0 | 0 | 0 | 0 | 2 (13) | 1 (7) |
| Hypomagnesemia | 0 | 0 | 5 (4) | 0 | 2 (13) | 0 | 2 (13) | 0 | 1 (7) | 0 |
| Influenza-like illness | 3 (8) | 0 | 3 (2) | 0 | 2 (13) | 0 | 1 (7) | 0 | 1 (7) | 0 |
| Musculoskeletal pain | 2 (5) | 0 | 3 (2) | 0 | 0 | 0 | 1 (7) | 0 | 2 (13) | 0 |
| Pain in extremity | 3 (8) | 0 | 9 (7) | 1 (1) | 2 (13) | 0 | 1 (7) | 0 | 1 (7) | 0 |
| Rash | 2 (5) | 0 | 10 (7.5) | 0 | 0 | 0 | 1 (7) | 0 | 2 (13) | 0 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MedDRA, Medical Dictionary for Regulatory Activities; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; TEAE, treatment-emergent adverse event.
Forty patients (30%) in the tumor-specific expansion cohorts experienced irAEs; the top irAEs were hypothyroidism, hyperthyroidism, colitis, and nephritis (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.102254). Eleven patients (8%) had grade 3 or 4 irAE reported, consisting of colitis (three patients, grade 3; one patient, grade 4), and acute kidney injury (grade 3), erythematous rash (grade 3), hypothyroidism (grade 3), maculopapular rash (grade 3), nephritis (grade 3), pneumonitis (grade 3), and type 1 diabetes mellitus (grade 3) in one patient each. Thirty-three patients (25%) had their dose interrupted and 19 patients (14%) discontinued study treatment due to TEAEs. Infusion-related reactions as per investigator were reported in 13 patients (10%). Four patients (3%) had a fatal TEAE in the tumor-specific expansion cohorts [cardiac failure and pulmonary embolism (one patient); cardiovascular insufficiency, hemiparesis, and nephritis (one patient each)]; none of the fatal events were attributed to retifanlimab by the investigator.
Patients received a range of 1-24 infusions of retifanlimab in the flat-dose cohorts; a median of 2 infusions was received by patients in the 500-mg q4w flat-dose cohort, and a median of 3 infusions in the 375-mg q3w and 750-mg q4w flat-dose cohorts. Among the 45 patients in the flat-dose cohorts, 43 (96%) experienced a TEAE; most common TEAEs were anemia [12 (27%)], fatigue [10 (22%)], and alkaline phosphatase increased [9 (20%)] (Table 2). TRAEs were reported by 25 patients (56%) (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102254); 14 patients (31%) experienced a serious TEAE. Treatment-emergent AESIs were reported by eight patients (18%) in the flat-dose cohorts, and six patients (13%) experienced irAEs (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.102254). The top irAEs were hypothyroidism and hyperthyroidism. One patient (2%) each reported a grade 3 AESI (amylase increased) and irAE (pneumonitis). Infusion-related reactions as per investigator were reported in two patients (4%), both at 750 mg q4w (one patient, grade 2; one patient, grade 1). Nine patients (20%) had dose interruption, and three patients (7%) discontinued study treatment due to TEAEs in the flat-dose cohorts. No fatal TEAEs occurred in the flat-dose expansion cohorts.
Antitumor activity
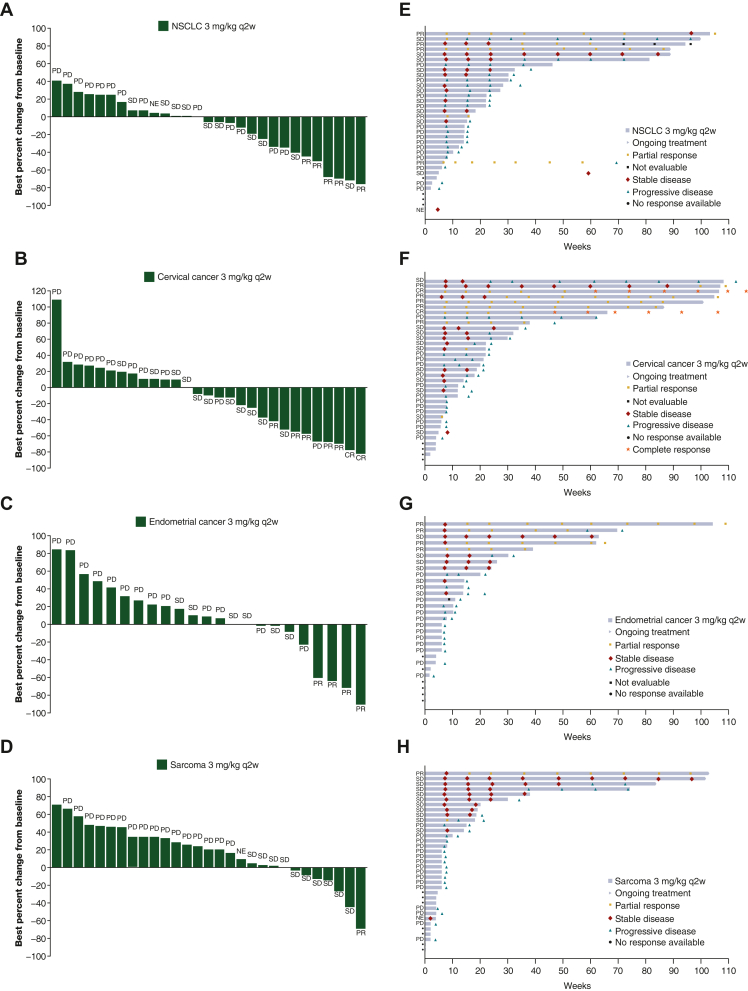
Of the 37 patients in dose escalation, three patients (8%) achieved an objective response {one confirmed PR each at dose level 3 mg/kg q2w, 3 mg/kg q4w, and 10 mg/kg q4w; tumor types were endometrial adenocarcinoma [microsatellite stable], squamous NSCLC [EGFR mutation], and colorectal carcinoma [microsatellite instability-high (MSI-H)], respectively}. In the tumor-specific expansion cohorts, ORR (95% CI) was 20.0% (8.4 to 36.9) in cervical cancer, 14.3% (4.8 to 30.3) in NSCLC, 13.8% (3.9 to 31.7) in biomarker-unselected endometrial cancer, and 2.9% (0.1 to 14.9) in soft tissue sarcoma. Clinical activity was representative of the PD-1 inhibitor class for all measures evaluated (Table 3, Figure 1). In the flat-dose tumor-agnostic cohorts, PRs were reported in one patient with breast cancer in the 500-mg q4w cohort and in two patients (one with endometrial adenocarcinoma and one with nasopharyngeal squamous cell carcinoma) in the 750-mg q4w cohort.
Table 3.
Summary of overall response in dose-escalation, tumor-specific, and flat-dose cohorts (efficacy-assessable population)
| Retifanlimab dose-escalation cohort (n = 37) | Tumor-specific cohorts (retifanlimab 3 mg/kg q2w) |
Flat-dose retifanlimab cohorts |
||||||
|---|---|---|---|---|---|---|---|---|
| NSCLC (n = 35) | Endometrial cancer (n = 29) | Cervical cancer (n = 35) | Soft tissue sarcoma (n = 35) | 375 mg q3w (n = 15) | 500 mg q4w (n = 15) | 750 mg q4w (n = 15) | ||
| ORR, n (%) | 3 (8) | 5 (14) | 4 (14) | 7 (20) | 1 (3) | 0 | 1 (7) | 2 (13) |
| 95% CI | 1.7-21.9 | 4.8-30.3 | 3.9-31.7 | 8.4-36.9 | 0.1-14.9 | 0-21.8 | 0.2-31.9 | 1.7-40.5 |
| Best overall response, n (%) | ||||||||
| CRa | 0 | 0 | 0 | 2 (6) | 0 | 0 | 0 | 0 |
| PRb | 3 (8) | 5 (14) | 4 (14) | 5 (14) | 1 (3) | 0 | 1 (7) | 2 (13) |
| SD | 10 (27) | 11 (31) | 6 (21) | 11 (31) | 10 (29) | 4 (27) | 5 (33) | 5 (33) |
| PD | 19 (51) | 14 (40) | 13 (45) | 13 (37) | 16 (46) | 10 (67) | 8 (53) | 6 (40) |
| NE | 0 | 1 (3) | 0 | 0 | 1 (3) | 0 | 0 | 0 |
| Missingc | 5 (14) | 4 (11) | 6 (21) | 4 (11) | 7 (20) | 1 (7) | 1 (7) | 2 (13) |
| Median PFS, months | 1.8 | 2.5 | 1.7 | 3.6 | 1.8 | 1.9 | 1.9 | 2.6 |
| 95% CI | 1.7-2.1 | 1.8-3.7 | 1.6-3.6 | 1.8-5.4 | 1.6-2.8 | 1.4-2.8 | 1.6-3.7 | 1.6-5.3 |
| Median OS, months | 6.6 | 8.8 | 11.6 | 18.1 | 9.7 | 13.4 | 7.8 | 10.9 |
| 95% CI | 3.6-10.5 | 5.9-20.9 | 6.2-17.4 | 12.1-24.5 | 5.1-11.2 | 2.6-14.1 | 2.2-13.3 | 3.5-NE |
| Median follow-up, months | 7.7 | 14.2 | 10.7 | 17.6 | 18.4 | NA | 3.7 | 4.5 |
| Range | 3.7-20.3 | 2.3-22.4 | 6.5-21.5 | 2.1-25.5 | 18.4-18.4 | 3.7-3.7 | 3.9-5.0 | |
CI, confidence interval; CPS, combined positive score; CR, complete response; MSI-H, microsatellite instability-high; MSS, microsatellite stable; NA, not available; NE, not evaluable; NSCLC, non-small-cell lung cancer; ORR, objective response rate; OS, overall survival; PD, progressive disease; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PR, partial response; q2w, every 2 weeks; q3w, every 3 weeks; q4w, every 4 weeks; SD, stable disease; TPS, tumor proportion score.
CR: cervical, PD-L1 CPS 13%, NA.d
PR: dose-escalation cohort cancer types colorectal (PD-L1 CPS 60%), endometrial (PD-L1 CPS 22%), NSCLC (PD-L1 TPS 80%); tumor-specific cohort NSCLC (PD-L1 TPS in five patients with PR: 0%, 55%, 65%, 85%, NAd), endometrial [biomarker status in four patients with PR: PD-L1 CPS 0%, 0%, 18%, NAd; MSI-H, MSS, NAd (two patients)], cervical [PD-L1 CPS in five patients with PR: 0%, 30%, 45%, NAd (two patients)], sarcoma cancer histology type undifferentiated pleomorphic (PD-L1 CPS 0%); flat-dose 500-mg q4w cohort, cancer type breast (PD-L1 CPS 0%); flat-dose 750-mg q4w cohort, cancer types endometrial adenocarcinoma (PD-L1 CPS 21%), nasopharyngeal squamous cell carcinoma (PD-L1 CPS 0%).
Patients had no postbaseline assessment available.
Insufficient tumor content available for testing.
Figure 1.
Change in target lesion size and duration of treatment in patients with solid tumors receiving retifanlimab. Waterfall plots represent best percentage change from baseline in target lesion size for individual patients receiving retifanlimab 3 mg/kg q2w in tumor-specific cohorts: (A) NSCLC, (B) cervical cancer, (C) endometrial cancer, and (D) soft tissue sarcoma. Swimlane plots represent duration of treatment and best objective responses for individual patients receiving retifanlimab 3 mg/kg q2w in tumor-specific cohorts: (E) NSCLC, (F) cervical cancer, (G) endometrial cancer, and (H) soft tissue sarcoma. Confirmed best objective response is shown for each patient in the figure. CR, complete response; NE, not evaluable; NSCLC, non-small-cell lung cancer; PD, progressive disease; PR, partial response; q2w, every 2 weeks; SD, stable disease.
Pharmacokinetics and immunogenicity
Retifanlimab first-dose pharmacokinetic parameters were available for 151 patients administered body weight-based doses (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102254) and 68 patients given flat doses (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.102254). Retifanlimab displayed approximately linear pharmacokinetics following first dose over all the dose ranges tested (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.102254); mean first-dose terminal half-life was ∼10.7 days (range 7.6-17.6 days). Accumulation of retifanlimab concentration was observed from cycle 2 to cycle 5, with apparent trough serum concentration at steady state at or before cycle 6. An accumulation ratio of ∼1.6 and ∼1.5 was observed with q2w and q3w body weight-based doses, and ∼1.3 with 500-mg q4w flat dose. Comparable steady-state trough concentration (Ctrough) values for the 3-mg/kg q2w, 375-mg q3w, and 500-mg q4w dose regimens were 44.0 ± 18.8 mg/l (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102254), 43.0 ± 15.0 mg/l, and 58.7 ± 26.8 mg/l (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.102254), respectively. The median steady-state retifanlimab Ctrough concentrations exceeded the target value of ≥21 μg/ml (based on pembrolizumab data14) in 62%, 58%, and 74% of patients at the respective doses of 375 mg q3w, 500 mg q4w, and 750 mg q4w.
Clinical immunogenicity was assessed in 36 patients in dose-escalation and 198 patients in dose-expansion cohorts. Two patients in the dose-expansion cohort were retifanlimab ADA positive at baseline. One patient each in the dose-escalation and cohort-expansion groups (and ADA negative at baseline) had treatment-emergent ADA. The dose-escalation cohort patient had received two infusions (retifanlimab 3 mg/kg q4w) and discontinued treatment (day 64) due to disease progression; the second infusion was interrupted because of a grade 2 infusion reaction. The cohort-expansion patient had received 50 infusions (retifanlimab 3 mg/kg q2w) and was ongoing on treatment at data cut-off; no infusion reactions were experienced.
Pharmacodynamics and exploratory translational analyses
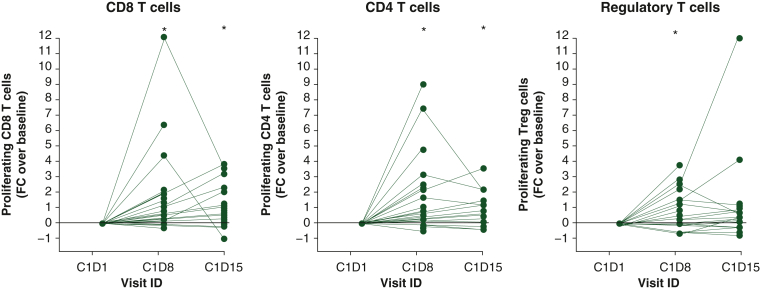
Retifanlimab demonstrated full PD-1 receptor occupancy on circulating CD8 T cells (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.102254), and increased serum chemokine C-X-C motif ligand 9 (CXCL9) and CXCL10 levels (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.102254), in both weight-based and flat-dosing cohorts. Retifanlimab administered at 3 mg/kg q2w and 500 mg or 750 mg q4w fully occupies PD-1 receptors on circulating CD4+ (data on file, Incyte Corporation, Wilmington, DE) and CD8+ T-cell subsets (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.102254), leading to increased interferon-γ-inducible protein levels (i.e. CXCL9 and CXCL10) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.102254), regardless of clinical outcomes and tumor type. Increased levels of proliferating (Ki67+) T cells in peripheral blood were observed, with peak values 8 days after infusion (Figure 2). Based on the pharmacokinetic profile, receptor occupancy, and pharmacodynamic data, the flat doses of 500 mg q4w and 375 mg q3w were selected for phase II studies.
Figure 2.
Increased proliferation in T cell subsets following treatment with retifanlimab. Fold-change in the frequency of proliferating (Ki67+) cells in different T-cell subsets (left panel: CD3+/CD8+; center panel: CD3+/CD4+; right panel: CD3+/CD4+/CD25+/FoxP3+). Analysis included 28 patients enrolled in the tumor-specific cohorts receiving retifanlimab 3 mg/kg q2w. ∗P < 0.05 by Wilcoxon matched pairs test with each time point compared with C1D1. C1D1, cycle 1 day 1; FC, fold change; q2w, every 2 weeks; Treg cells, regulatory T cells.
Tumor PD-L1 expression and MSI status were examined retrospectively when baseline biopsy specimens were available; as expected, the ORR was greater in patients with documented high PD-L1 tumor expression (data on file, Incyte Corporation). The number of MSI-H tumors was too limited for comparisons to be made. Sequencing analysis of baseline tumor biopsy specimens indicated that clinical response to retifanlimab was associated with an inflamed RNA signature (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.102254).
Discussion
This phase I dose-escalation and dose-expansion study evaluated the anti-PD-1 antibody, retifanlimab, in patients with relapsed/refractory, unresectable, locally advanced or metastatic solid tumors. Retifanlimab was generally well tolerated and showed clinical pharmacology consistent with the PD-1 inhibitor class. In the dose-escalation cohort, there were no DLTs reported and no MTD was defined. The MAD was 10 mg/kg, and a retifanlimab dose of 3 mg/kg was selected for dose expansion in tumor-specific cohorts.
Additional exploration of flat dosing was carried out, as this confers significant advantages over body weight-based dosing, including convenience, cost-effectiveness, and lower risk of dosing errors.15 Based on a consistent pharmacokinetic profile, full and sustained receptor occupancy of retifanlimab on both CD4+ and CD8+ T cells along with complete loss of competing fluorescently labeled anti-PD-1 staining seen at all dose levels (providing pharmacodynamic support of biological response to retifanlimab), a comparable safety profile to that of 3 mg/kg q2w, and tumor responses observed in all tumor-specific cohorts at 3 mg/kg q2w and at 500 mg q4w and 750 mg q4w, the flat doses of 500 mg q4w and 375 mg q3w were selected for phase II and phase III studies.
Safety was entirely representative of what has previously been reported for the PD-(L)1 inhibitor class.16, 17, 18 Our study included patients with recurrent/refractory advanced solid tumors who received up to five prior systemic therapies, with 50% of patients having received more than two prior chemotherapy-based regimens. In this moderate to heavily pretreated population, 3 (8.1%) and 24 (10.8%) patients in the dose-escalation and dose-expansion cohorts, respectively, received at least 1 year of treatment and the safety and toxicity profile of retifanlimab at all dose levels was consistent with that expected from a PD-1 targeting CPI. Overall, TEAEs were predominantly of mild to moderate severity and manageable with standard of care. Grade ≥3 TEAEs were reported in 73% and 46% of patients in dose escalation and cohort expansion, respectively. The incidence of TEAEs leading to retifanlimab discontinuation was low (no patient in dose escalation and 12% of patients in cohort expansion).
The incidence and severity of irAEs and infusion reactions in this study were as expected for the PD-(L)1 inhibitor class.19 Among the 179 patients in cohort expansion, irAEs occurred in 26% and grade 3 or 4 irAEs occurred in 7% of patients. These incidences are similar to those from a meta-analysis of 46 PD-1/PD-L1 inhibitor studies that showed incidences of 26.8% (95% CI 21.7 to 32.6) for any-grade irAEs and 6.1% (95% CI 4.9 to 7.6) for severe irAEs.16 The most frequent irAEs in cohort expansion were hypothyroidism (9%), which was grade 1 or 2 in severity in all but one patient (1%); hyperthyroidism (7%) of grade 1 or 2; and colitis (3%), which was grade 2 in one patient (1%), grade 3 in three patients (2%), and grade 4 in one patient (1%). irAEs were not dose related and were manageable with standard-of-care measures.20 Infusion reactions were uncommon and not clinically significant. Based on these results, premedication prophylaxis is not required for routine administration of retifanlimab. The incidence of treatment-emergent ADA was also low (2 of 216 assessable patients).
Retifanlimab elicited durable, confirmed responses in patients with various advanced solid tumor types, including tumors with limited treatment options in the second-line setting and beyond. Confirmed responses were achieved in moderate to heavily pretreated patients in all tumor-specific cohorts and compare well with established PD-(L)1 inhibitors.4,5,21 Treatment of MSI-H or deficient mismatch repair endometrial cancer with retifanlimab 500 mg q4w has also shown encouraging results in another ongoing expansion cohort, further supporting the clinical activity and ongoing investigation in this tumor type.22,23 Gene expression (RNAseq) analysis of baseline tumor biopsies and analysis of T-cell infiltration by multiplex IHC (data on file, Incyte Corporation) demonstrated that CD8+ T-cell infiltration, and an inflamed RNA signature, was associated with clinical responses to retifanlimab. This is in accordance with previous reports for PD-1-targeted therapy.24,25 Translational data also confirmed that retifanlimab was biologically active in all evaluated dose regimens, including increased expression of CD3+ T-cell activation markers (CD38, CD278), and up-regulated serum levels of interferon-γ-inducible chemokines (CXCL9, CXCL10).26
In conclusion, retifanlimab was generally well tolerated in patients with pretreated recurrent/refractory, unresectable, locally advanced or metastatic solid tumors. Retifanlimab demonstrated favorable pharmacokinetics and receptor occupancy, and dosing schedules of 375 mg q3w and 500 mg q4w have been optimized for clinical use, both as monotherapy and in potential future novel combinations. Observed irAEs were consistent with the extensive clinical experience for PD-1/PD-L1 inhibitors.19 Preliminary clinical activity in advanced and recurrent NSCLC, cervical, and endometrial tumor-specific cohorts is encouraging, and consistent with previously reported activity of other PD-(L)1-directed therapies.27, 28, 29, 30 As such, retifanlimab is currently being investigated in clinical trials as a potential therapeutic option for multiple solid tumor types, both as a monotherapy and in combination with other immunotherapies and chemotherapy. Recently, retifanlimab was granted accelerated approval by the US Food and Drug Administration for adult patients with metastatic or recurrent locally advanced Merkel cell carcinoma.31
Ethics approval and consent to participate
The study was approved by institutional review boards or independent ethics committees in Australia (St Vincent’s Hospital Sydney Research Office, Darlinghurst); Belgium (Aan de Commissie Medische Ethiek UZ Leuven; Ethics Committee of Hospital-Faculty University of Liège); Bulgaria (Ethics Committee for Clinical Trials, Sofia); Finland (HUS Tutkimuseettiset Toimikunnat Biomedicum Helsinki); France (CPP Île-de-France X Hôpital Robert Ballanger, Aulnay-sous-Bois cedex); Germany (Ethics Committee of the State of Berlin; Ethics Committee of the Bavarian Medical Association, München; Ethik-Kommission der Albert-Ludwigs-Universität Freiburg; Ethics Committee at the Technical University of Dresden); Italy [Comitato Etico del Policlinico Gemelli Fondazione Policlinico Universitario “Agostino Gemelli”, Roma (RM); Comitato Etico IRCCS di Candiolo, Candiolo-TO]; Latvia (Ethics Committee for Clinical Research at Development Society of Pauls Stradins Clinical University Hospital, Riga); Lithuania (Lithuanian Bioethics Committee, Vilnius); New Zealand (Northern B Health and Disability Ethics Committee, Wellington); Poland (Komisja Bioetyczna przy Uniwersytecie Medycznym, Poznań); Spain (Comité de Ética de Investigación con Medicamentos, Madrid Centro); Ukraine (Ethical Committee at City Clinical Hospital #4 of Dnipro City Rada, Dnipro; Ethical Committee at Uzhgorod Central City Clinical Hospital of Uzhgorod Regional Rada, Uzhgorod; Ethical Committee at Prykarpatsky Regional Clinical Oncology Center of Ivano-Frankivsk Regional Rada, Ivano-Frankivsk; Ethical Committee at Sumy Clinical Oncological Dispensary of Sumy Regional Rada, Sumy; Ethics Committee at the Municipal Non-profit Enterprise “Podillia Regional Oncology Center of Vinnytsia Regional Council”, Vinnytsia); United Kingdom [Research Ethics Committee (REC) London Centre)]; United States [Advarra IRB, Columbia, MD; IntegReview IRB, Austin, TX; Western Institutional Review Board (WIRB), Puyallup, WA; U.T. MD Anderson Cancer Center Institutional Review Board, Houston, TX]. The study was conducted following International Council for Harmonisation Guidelines for Good Clinical Practice, and all other local applicable regulations. All patients provided written informed consent before enrolling in the study.
Acknowledgements
The authors wish to thank the patients, investigators, and site personnel who participated in this study. The authors would like to thank Monika Dudzisz-Sledz (KCR S.A., Warsaw, Poland, Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland), Thomas Condamine (formerly at Incyte Corporation), and MacroGenics, Inc. for their contributions to the study, as well as Sulabha Ranganathan for clinical review and Yubing Yao for statistical analysis support. The UK centers acknowledge infrastructural support from Cancer Research UK, the Experimental Cancer Medicine Centre, and Biomedical Research Centre initiatives. Medical writing assistance was provided by Matthew Bidgood, PhD, of Envision Pharma Group (Philadelphia, PA), funded by Incyte Corporation.
Data sharing
Access to individual patient-level data is not available for this study.
Funding
This study was sponsored by Incyte Corporation (Wilmington, DE, USA) (no grant number). Medical writing was also funded by Incyte Corporation. The UK centers received National Institute of Health and Care Research Biomedical Research Centre funding (no grant number).
Disclosure
NL reports consulting or advisory roles with Ikena, Innovent Biologics, S.K. Life Sciences; research funding from Alexion Pharmaceuticals, Alexo Therapeutics, Alkermes, Alpine Biosciences, Alpine Immune Sciences, Apexian Pharmaceuticals, Asana Biosciences, Ascentage Pharma, Astellas Pharma, BeiGene, Celgene, Cerulean Pharma, Constellation Pharmaceuticals, Coordination Therapeutics, CytomX Therapeutics, Epizyme, Formation Biologics, Forty Seven, Genmab, Gilead, GlaxoSmithKline, Helsinn Therapeutics, Ikena, Ikena Oncology, Incyte, InhibRx, Innovent Biologics, Jounce Therapeutics, LAM Therapeutics, Lilly, Livzon, Loxo, Macrogenics, Merck, Mersana, Northern Biologics, Odonate, Pfizer, Regeneron Pharmaceuticals, Inc., Sapience Therapeutics, Seagen, Servier, Shattuck Labs, Symphogen, Tesaro, Tizona. RC reports employment at The Kinghorn Cancer Centre; principal investigator on a number of phase I clinical trials. UB reports honoraria from Astellas, Boehringer Ingelheim, Janssen, Karus Therapeutics, Novartis, Pegascy; funding for phase I investigator-initiated trials with AstraZeneca, BTG International, Carrick Therapeutics, Chugai, Onyx Pharmaceuticals, Verastem; employment at Institute of Cancer Research, London, which is involved in the development of AKT, CHK1, HDAC, HSF1, HSP90, PI3K, RAF, and ROCK inhibitors. DR reports research funding from Incyte Corporation. MT-K reports employment at BioVirtus during the conduct of the study; BioVirtus received financial compensation from Incyte for the clinical conduct. EG reports personal fees from Alkermes, Anaveon, Boehringer Ingelheim, Bristol Meyers Squibb, Ellipses Pharma, F. Hoffmann/La Roche Ltd, F-Star Therapeutics, Hengrui, Janssen Global Services, MabDiscovery, Neomed Therapeutics 1 Inc., Roche/Genentech, SeaGen, TFS HealthScience, and Thermo Fisher; personal fees from Lilly, Merck Sharp & Dohme Merck Sharp & Dohme, Novartis, Roche, and Thermo Fisher; grants from AstraZeneca, BeiGene, Novartis, Roche, Taiho, and Thermo Fisher; other support from Agios Pharmaceuticals, Amgen, Bayer, Beigene USA, Blueprint Medicines, Bristol Meyers Squibb, Cellestia Biotech, Debiopharm, F. Hoffmann/La Roche Ltd, Forma Therapeutics, Genentech Inc., Genmab B.V., GlaxoSmithKline, Glycotope Gmbh, Incyte Biosciences, Incyte Corporation, ICO, Kura Oncology Inc, Lilly, S.A, Loxo Oncology Inc, Macrogenics Inc, Menarini Ricerche Spa, Merck Sharp & Dohme de España, S.A, Nanobiotix, S.A., Novartis Farmacéutica, S.A., Pfizer, SLU, Pharma Mar, S.A.U., Pierre Fabre Medicament, Principia Biopharma Inc., Psioxus Therapeutics Ltd, Sanofi, Sierra Oncology, Inc., Sotio A.S., and Symphogen A/S. DK and CT report employment and stock ownership with Incyte Corporation. JL reports former employment and stock ownership with Incyte Corporation. NB reports employment and stock ownership with Incyte Biosciences International Sàrl. JP reports intellectual property rights/patent holder with BioCytics Inc.; consulting fees from Aavocyte, AbbVie, Affivant, Boxer Capital, Macrogenics, Phanes Therapeutics, TBP Therapeutics, Top Alliance; contracted research for Aavocyte, AbbVie, Adagene, Alkermes, Apros, Arcus BioSciences, AstraZeneca, Atreca, BJ BioScience, Bristol Myers Squibb, Calico Life Sciences, Conjupro BioTherapeutics, CUE BioPharma, Cullinan, EMD Serono, FLX Bio/RAPT Therapeutics, Genentech/Roche, IMAB Pharma, Immune-Onc, Incyte, Jounce Therapeutics, Macrogenics, Medimmune, Merck, Moderna TX, Molecular Templates, MT Group, NexCure, Nuvation, PEEL Therapeutics, Pieris, PIOMA, Precision for Medicine, Repertoire Immune Medicines, Replimmune, RiboScience, Seattle Genetics, Sequenom, StemCell Technologies, Tempest Therapeutics, Top Alliance BioScience, Trethera, Xilio Therapeutics, Xilis, Zenshine Pharma; ownership interest in BioCytics Inc, Carolina BioOncology Institute PLLC; other relationships: BioCytics is developing intellectual property for point of care cell therapies.
Supplementary data
References
- 1.Paluch C., Santos A.M., Anzilotti C., Cornall R.J., Davis S.J. Immune checkpoints as therapeutic targets in autoimmunity. Front Immunol. 2018;9:2306. doi: 10.3389/fimmu.2018.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan C., Liu H., Robins E., et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol. 2020;13(1):29. doi: 10.1186/s13045-020-00862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipson E.J., Drake C.G. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17(22):6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu-Monette Z.Y., Zhang M., Li J., Young K.H. PD-1/PD-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol. 2017;8:1597. doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall H.T., Djamgoz M.B.A. Immuno-oncology: emerging targets and combination therapies. Front Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golay J., Andrea A.E. Combined anti-cancer strategies based on anti-checkpoint inhibitor antibodies. Antibodies (Basel) 2020;9(2):17. doi: 10.3390/antib9020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi A.-P., Tang X.-Y., Xiong Y.-L., et al. Immune checkpoint LAG3 and its ligand FGL1 in cancer. Front Immunol. 2022;12 doi: 10.3389/fimmu.2021.785091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Motte-Mohs R., Shah K., Brown J.G., et al. Preclinical characterization of MGA012, a novel clinical-stage PD-1 monoclonal antibody [abstract P336] J Immunother Cancer. 2017;5(suppl 2):87. [Google Scholar]
- 11.Lakhani N., Mehnert J.M., Rasco D., et al. A phase 1 study of the safety, tolerability, and pharmacokinetics (PK) of MGA012 (anti-PD-1 antibody) in patients with advanced solid tumors [abstract P249] J Immunother Cancer. 2017;5(suppl 2):87. [Google Scholar]
- 12.Mehnert J.M., Joshua A., Lakhani N., et al. First-in-human phase 1 study of INCMGA00012 in patients with advanced solid tumors: interim results of the cohort expansion phase [abstract P669] J Immunother Cancer. 2018;6(suppl 1):115. [Google Scholar]
- 13.Mehnert J.M., Ares L.P., Pikiel J., et al. A phase 1 study of INCMGA00012, a PD-1 inhibitor, in patients with advanced solid tumors: preliminary results for patients with advanced cervical cancer (POD1UM-101) [abstract P394] J Immunother Cancer. 2019;7(suppl 1):282. [Google Scholar]
- 14.Freshwater T., Kondic A., Ahamadi M., et al. Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer. 2017;5:43. doi: 10.1186/s40425-017-0242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D.D., Zhang S., Zhao H., Men A.Y., Parivar K. Fixed dosing versus body size–based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49(9):1012–1024. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- 16.Wang P.F., Chen Y., Song S.Y., et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C., Chen Y.P., Du X.J., et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ. 2018;363:k4226. doi: 10.1136/bmj.k4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L., Sun L., Zhou Y., et al. Association of survival and immune-related adverse events with anti-PD-1/PD-L1 and anti-CTLA-4 inhibitors, alone or their combination for the treatment of cancer: a systematic review and meta-analysis of 13 clinical trials. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.575457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fessas P., Possamai L.A., Clark J., et al. Immunotoxicity from checkpoint inhibitor therapy: clinical features and underlying mechanisms. Immunology. 2020;159(2):167–177. doi: 10.1111/imm.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network Management of Immunotherapy-Related Toxicities (Version 2.2023) 2023. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf Available at.
- 21.Sun L., Zhang L., Yu J., et al. Clinical efficacy and safety of anti-PD-1/PD-L1 inhibitors for the treatment of advanced or metastatic cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):2083. doi: 10.1038/s41598-020-58674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berton-Rigaud D., Vergote I., Pautier P., et al. A phase 1 study of retifanlimab (INCMGA00012), a PD-1 inhibitor, in patients with advanced solid tumors: preliminary results in recurrent MSI-high or dMMR endometrial cancer (POD1UM-101) [abstract 268] J Immunother Cancer. 2020;8(suppl 3):A294. [Google Scholar]
- 23.Berton D., Pautier P., Lorusso D., et al. Retifanlimab (INCMGA00012) in patients with recurrent MSI-H or dMMR endometrial cancer: results from the POD1UM-101 study [abstract 956] J Immunother Cancer. 2021;9(suppl 2):A1006. [Google Scholar]
- 24.Ayers M., Lunceford J., Nebozhyn M., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristescu R., Mogg R., Ayers M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condamine T., Owens S., Feldman P., et al. Pharmacodynamic correlates in a phase I study of INCMGA00012, a PD-1 antagonistic monoclonal antibody [abstract] Cancer Res. 2019;79(suppl 13):CT085. [Google Scholar]
- 27.Di Tucci C., Capone C., Galati G., et al. Immunotherapy in endometrial cancer: new scenarios on the horizon. J Gynecol Oncol. 2019;30(3):e46. doi: 10.3802/jgo.2019.30.e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagabu M., Nagasawa T., Sato C., et al. Immunotherapy for uterine cervical cancer using checkpoint inhibitors: future directions. Int J Mol Sci. 2020;21(7):2335. doi: 10.3390/ijms21072335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S.Y., Halmos B. Choosing the best first-line therapy: NSCLC with no actionable oncogenic driver. Lung Cancer Manag. 2020;9(3):Lmt36. doi: 10.2217/lmt-2020-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasser N.J., Gorenberg M., Agbarya A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals. 2020;13(11):373. doi: 10.3390/ph13110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US F.D.A. ZYNYZ™ (retifanlimab-dlwr) injection, for intravenous use. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761334Orig1s000correctedlbl.pdf Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.