Abstract
A 63-year-old woman presented with hypokalemia, hypertension, weight gain, limb edema, and tremors. She was diagnosed with Cushing syndrome, with a 24-hour urine cortisol level of 41,013 nmol/day. Investigations revealed a grade 2 pancreatic neuroendocrine tumor with extensive hepatic metastases. Owing to excessive adrenocorticotropic hormone production from her disease, her hypercortisolemia and Cushing symptoms worsened despite ketoconazole, somatostatin analogs, and right liver lobe chemoembolization. Stereotactic body radiotherapy (SBRT) at a dose of 39 Gy in three fractions was administered to her bilateral adrenal glands in the hope of reducing her cortisol levels and improving her symptoms. Her 24-hour urine cortisol levels decreased following SBRT, but not rapidly enough; her clinical condition continued to deteriorate, and she died 21 days after treatment. SBRT was not effective as an urgent intervention in this setting; a greater latency to realize a response is likely necessary.
Keywords: stereotactic body radiotherapy, pancreatic neoplasms, neuroendocrine tumors, hypercortisolism, ectopic acth syndromes, adrenal glands
Introduction
Pancreatic neuroendocrine tumors (PNETs) account for approximately 2% of pancreatic tumors [1]. PNETs can be classified histologically as well-differentiated and poorly differentiated and clinically as functioning and nonfunctioning. Functioning tumors represent approximately 30% of PNETs and include insulinomas, gastrinomas, glucagonomas, somatostatinomas, vasoactive intestinal peptide tumors, and adrenocorticotropic hormone (ACTH)-producing tumors [2,3].
ACTH-secreting PNETs are uncommon; they are usually associated with liver metastases that develop prior to patients noticing symptoms, and they can be associated with a poor prognosis [4-7]. High ACTH levels can result in hypercortisolemia and Cushing syndrome, with its attendant neuropsychiatric, metabolic, dermatologic, musculoskeletal, and cardiovascular symptoms [8].
Initial management of Cushing syndrome can include ketoconazole and somatostatin analogs [9-12]. If symptoms persist despite pharmacologic therapy, local therapy options can be considered. In the setting of a metastatic PNET, these can include resection of the primary tumor and/or bulky liver metastases, chemoembolization of liver metastases, adrenalectomy, and adrenal radiofrequency ablation.
If such options are not possible or prove ineffective, an alternative nonsurgical local treatment could be adrenal ablation with stereotactic body radiotherapy (SBRT). Stereotactic radiotherapy is a standard treatment option for functioning pituitary tumors, including those that secrete ACTH [13,14]. To our knowledge, however, the use of adrenal SBRT to manage hypercortisolemia secondary to an ACTH-secreting PNET has not been reported. There is a known iatrogenic risk of adrenal insufficiency months following bilateral adrenal tumor irradiation otherwise [15] (e.g., in the setting of aggressive treatment for oligometastatic cancers); however, whether the latency to insufficiency following planned comprehensive bilateral adrenal SBRT would be too long to help in the setting of worsening Cushing syndrome symptoms is an open question.
We report here a case of attempted symptom control with bilateral adrenal SBRT in a patient with treatment-refractory Cushing syndrome secondary to an ACTH-secreting metastatic PNET.
Case presentation
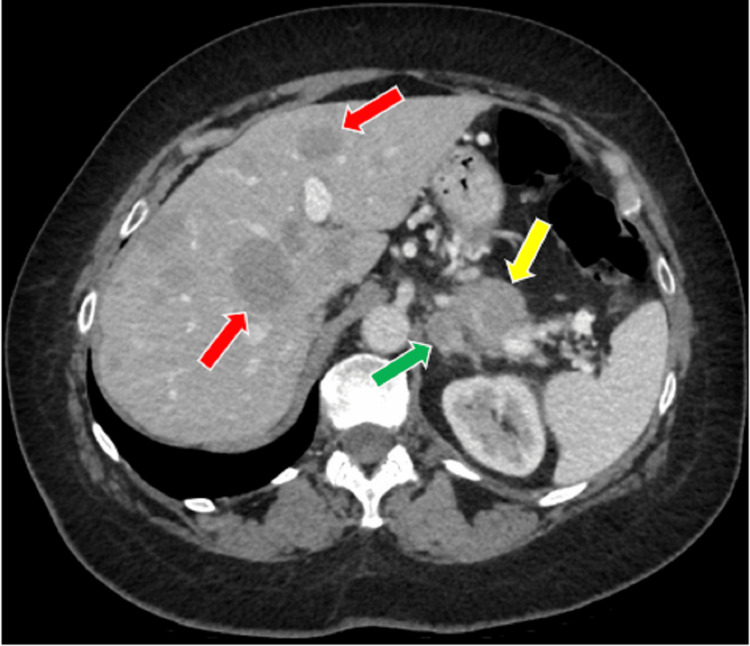
A previously healthy 63-year-old woman presented with a three-week history of unexplained hypertension, weight gain from 183 to 200 pounds, bilateral upper limb edema, and whole-body tremors occurring approximately six times daily and lasting between five and 10 minutes. On assessment in the emergency room, a blanchable maculopapular rash was seen on her neck, arms, and legs. Her bloodwork showed hypokalemia (2.5 nmol/L, reference (ref) range: 3.5-5.1) with metabolic alkalosis, an elevated ACTH (105 pmol/L, ref range: 1.6-13.9), hypercortisolemia (2,587 nmol/L, ref range: 68-327), and mildly elevated liver enzymes (aspartate aminotransferase: 39 U/L, ref range: 12-29; alanine aminotransferase: 65 U/L, ref range: 8-33). She was admitted with suspected Cushing syndrome, and a 24-hour urine cortisol test confirmed this with a level of 41,013 nmol/day (ref range: 60-415). CT of the head, chest, abdomen, and pelvis showed a 3.5-cm pancreatic tail mass suspicious for a primary tumor, a 1.7-cm left adrenal nodule, and innumerable hepatic metastases (Figure 1). An ultrasound-guided biopsy of a liver metastasis confirmed a grade 2 neuroendocrine tumor with a Ki67 of 6% and cells staining positive for cytokeratin 8/18, chromogranin A, and inhibin and negative for synaptophysin and P40.
Figure 1. CT axial plane image of left adrenal mass (green arrow) adjacent to primary pancreatic mass (yellow arrow) and hepatic metastases (red arrows).
The patient was treated with potassium replacement and spironolactone to manage the apparent mineralocorticoid excess, ketoconazole to reduce cortisol production, and once pathology confirmed a PNET, short-acting and eventually long-acting somatostatin analogs were added. However, her symptoms due to hypercortisolemia persisted, and she ultimately agreed to a right liver lobe portal vein embolization in the hope of reducing the amount of ectopic ACTH being secreted from her hepatic metastases. She was discharged the day following embolization, approximately three weeks from her initial admission, with her 24-hour urine cortisol level reduced from baseline at 22,982 nmol/day (Table 1).
Table 1. Serum and 24-hour urine cortisol levels over time.
KTC, ketoconazole; SBRT, stereotactic body radiotherapy; SSA, somatostatin analog
| Time point | Description | Serum cortisol (nmol/L) | 24-hour urine cortisol (nmol/d) |
| Day 0 | Initial presentation and admission | 2,587 | |
| Day 2 | 41,013 | ||
| Day 19 | After KTC and SSAs | 22,982 | |
| Day 23 | Discharge | 3,448 | |
| Day 39 | 18 days after liver embolization | 14,216 | |
| Day 45 | Readmission | 6,127 | |
| Day 50 | 4 days prior to SBRT | 6,507 | |
| Day 59 | 1 day after SBRT | 5,855 | |
| Day 60 | 2 days after SBRT | 4,643 | |
| Day 61 | 3 days after SBRT | 5,523 | |
| Day 64 | 6 days after SBRT | 6,193 | |
| Day 67 | 9 days after SBRT | 7,595 | |
| Day 68 | 10 days after SBRT | 10,300 | |
| Day 70 | 12 days after SBRT | 8,206 |
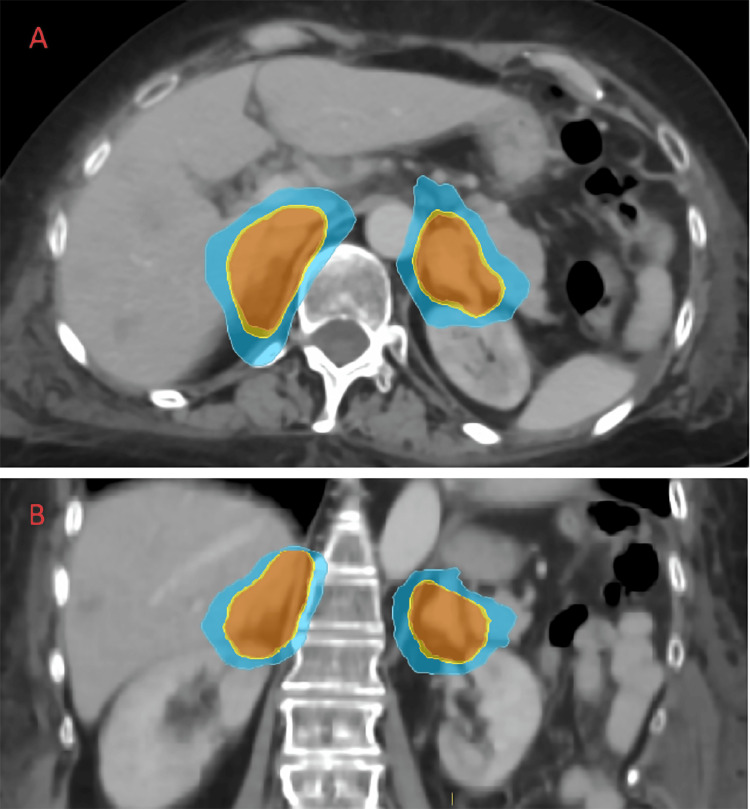
The patient was admitted again approximately three weeks later with persistent symptoms of Cushing syndrome, including significant depression. Her 24-hour urine cortisol level was 14,216 nmol/day. In the hope of further improving her hypercortisolism, she agreed to a course of SBRT for both adrenal glands at a dose of 39 Gy in three fractions, administered on consecutive days (Figure 2). She tolerated treatment well without troublesome acute toxicities. In tracking her biochemical response to treatment, we observed a difference between serum and urine cortisol levels (Table 1). Four days prior to SBRT, her serum cortisol was 6,507 nmol/L. It reached a nadir of 4,643 nmol/L two days after SBRT completion but then rose to 8,206 nmol/L 12 days after SBRT. By comparison, her 24-urine cortisol level (a more reliable indicator of treatment response) 10 days after SBRT had dropped from its pretreatment level to 10,300 nmol/day. Unfortunately, despite this incremental improvement, by that time her depression had worsened, she was having intermittent bouts of symptomatic hypotension, she had developed significant upper and lower limb edema, and her blood cultures had grown Escherichia coli from a presumed urinary source. A decision was made to focus on comfort care alone, and she died 21 days after SBRT, less than three months after her initial presentation. An autopsy was declined by the family.
Figure 2. Radiotherapy treatment planning CT axial plane image (A) and coronal plane image (B) showing isodose levels overlying the adrenal glands: 3,900 cGy/100% (orange), 3,705 cGy/95% (yellow), 3,000 cGy/77% (green), and 2,730 cGy/70% (blue).
Discussion
This is the first case report of which we are aware that describes the use of bilateral adrenal SBRT in the hope of controlling symptoms from hypercortisolemia in the setting of an otherwise treatment-refractory PNET causing excessive ACTH production.
The treatment of ectopic ACTH can be challenging, and management should be individualized and guided by multidisciplinary collaboration. Guidelines and expert opinions exist for the management of Cushing syndrome due to ectopic ACTH, but owing to the very heterogeneous affected patient phenotypes, no simple algorithm can be followed [16-18]. In general, if medical therapy cannot adequately control the symptoms of a metastatic PNET, local therapy options such as adrenalectomy or adrenal radiofrequency ablation can be considered, along with surgical resection of the primary and/or hepatic metastases or chemoembolization of extensive areas of hepatic metastases. Given our patient’s poor performance status and personal wishes, along with the extensive distribution of her disease, after her chemoembolization procedure, nonsurgical therapies were pursued. Prognostic models for affected patients could potentially assist in clinical decision-making, and some cohort studies have found that certain features such as hypokalemia and acute onset are associated with worse outcomes [17,18], but again, owing to the heterogeneous patient presentations, formal prognostication tools are still lacking.
The SBRT prescription administered to our patient (39 Gy in three fractions; biologically equivalent dose (BED10) of 89.7 Gy) was in line with those reported in a series of SBRT regimens of different durations and doses for adrenal metastases elsewhere [19,20]. Owing to her declining performance status, a short fractionation schedule was desired. A systematic review of 39 studies with 1,006 patients who received SBRT for adrenal metastases (66% of patients had lung cancer) suggested a possible dose response with regimens with a BED10 >100 Gy potentially providing better local control; however, adrenal toxicities were rarely addressed in the contributing studies, with only five total patients from two studies being listed as developing grade 2 adrenal insufficiency [16]. Whether higher BED regimens within the SBRT range predict greater impairment of adrenal function is an open question.
A single-institution study published after the systematic review described findings from 56 patients who had undergone adrenal SBRT for metastatic disease and had ongoing assessments by an endocrinologist [15]. Among these carefully monitored patients, 80% of those who received treatment for both glands (or their solitary remaining gland) developed primary adrenal insufficiency with a median time of onset of six months following SBRT (range: 0.5-20.4 months). This suggests that, by extrapolation, using bilateral SBRT for symptom control in a setting such as the one we report here may not, on its own, provide rapid improvements in hypercortisolemia and Cushing symptoms. Continued bridging medical therapy is likely necessary while waiting for the effect of SBRT to be realized, and using SBRT earlier in a patient’s treatment course should be considered.
Our case also highlights the importance of monitoring treatment response not only with point-of-care serum cortisol levels but also with more reliable 24-hour urine cortisol levels. Despite declining urinary levels after both the liver embolization and later SBRT, in the 21 days following SBRT during which our patient was living, serum levels steadily increased. Monitoring only serum levels can lead to misinterpretations of treatment effectiveness in this setting.
Conclusions
We report here a unique case of bilateral adrenal SBRT administered in the hope of controlling symptoms from hypercortisolemia in the setting of an otherwise treatment-refractory metastatic PNET causing excessive ACTH production. Our patient’s cortisol levels improved over time following sequential therapies including SBRT but remained significantly elevated, leading to clinical decline and death 21 days following SBRT. Practitioners with similarly affected patients should likely expect a delayed response to SBRT in this setting and not rely on it solely to induce rapid clinical improvements.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Kristopher Dennis, Said Al Saifi, Michael Vickers, Irena Druce
Acquisition, analysis, or interpretation of data: Kristopher Dennis, Said Al Saifi, Michael Vickers, Irena Druce
Drafting of the manuscript: Kristopher Dennis, Said Al Saifi
Critical review of the manuscript for important intellectual content: Kristopher Dennis, Michael Vickers, Irena Druce
Supervision: Kristopher Dennis
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Pancreatic neuroendocrine neoplasms: 2020 update on pathologic and imaging findings and classification. Khanna L, Prasad SR, Sunnapwar A, et al. Radiographics. 2020;40:1240–1262. doi: 10.1148/rg.2020200025. [DOI] [PubMed] [Google Scholar]
- 2.Neoplasms of the neuroendocrine pancreas: an update in the classification, definition, and molecular genetic advances. Guilmette JM, Nosé V. Adv Anat Pathol. 2019;26:13–30. doi: 10.1097/PAP.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 3.A clinicopathologic and molecular update of pancreatic neuroendocrine neoplasms with a focus on the new World Health Organization classification. Fang JM, Shi J. Arch Pathol Lab Med. 2019;143:1317–1326. doi: 10.5858/arpa.2019-0338-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACTH-secreting neuroendocrine pancreatic tumor: a case report. Surace A, Ferrarese A, Benvenga R, et al. Int J Surg. 2014;12 Suppl 1:0–4. doi: 10.1016/j.ijsu.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 5.A case of ectopic adrenocorticotropic hormone-producing pancreatic neuroendocrine tumor with multiple liver metastases. Kondo T, Matsuyama R, Ashihara H, et al. Endocr J. 2010;57:229–236. doi: 10.1507/endocrj.k09e-179. [DOI] [PubMed] [Google Scholar]
- 6.Mortality in Cushing's syndrome: data from 386 patients from a single tertiary referral center. Yaneva M, Kalinov K, Zacharieva S. Eur J Endocrinol. 2013;169:621–627. doi: 10.1530/EJE-13-0320. [DOI] [PubMed] [Google Scholar]
- 7.Adrenocorticotropic hormone--secreting islet cell tumors: are they always malignant? Doppman JL, Nieman LK, Cutler GB Jr, Chrousos GP, Fraker DL, Norton JA, Jensen RT. Radiology. 1994;190:59–64. doi: 10.1148/radiology.190.1.8259429. [DOI] [PubMed] [Google Scholar]
- 8.Complications of cushing's syndrome: state of the art. Pivonello R, Isidori AM, De Martino MC, et al. Lancet Diabetes Endocrinol. 2016;4:611–629. doi: 10.1016/S2213-8587(16)00086-3. [DOI] [PubMed] [Google Scholar]
- 9.Ketoconazole in the management of paraneoplastic Cushing's syndrome secondary to ectopic adrenocorticotropin production. Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. J Clin Oncol. 1995;13:157–164. doi: 10.1200/JCO.1995.13.1.157. [DOI] [PubMed] [Google Scholar]
- 10.Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. Biller BM, Grossman AB, Stewart PM, et al. J Clin Endocrinol Metab. 2008;93:2454–2462. doi: 10.1210/jc.2007-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Understanding the management and treatment of well-differentiated pancreatic neuroendocrine tumors: A clinician's guide to a complex illness. Li D, Rock A, Kessler J, et al. JCO Oncol Pract. 2020;16:720–728. doi: 10.1200/JCOOP.20.00010. [DOI] [PubMed] [Google Scholar]
- 12.Treatment options for pancreatic neuroendocrine tumors. Akirov A, Larouche V, Alshehri S, Asa SL, Ezzat S. Cancers (Basel) 2019;11:828. doi: 10.3390/cancers11060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stereotactic radiosurgery for secretory pituitary adenomas: systematic review and International Stereotactic Radiosurgery Society practice recommendations. Mathieu D, Kotecha R, Sahgal A, et al. J Neurosurg. 2022;136:801–812. doi: 10.3171/2021.2.JNS204440. [DOI] [PubMed] [Google Scholar]
- 14.Stereotactic radiotherapy and radiosurgery for non-functioning and secreting pituitary adenomas. Minniti G, Clarke E, Scaringi C, Enrici RM. Rep Pract Oncol Radiother. 2016;21:370–378. doi: 10.1016/j.rpor.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radiation of bilateral adrenal metastases is associated with a high risk of primary adrenal insufficiency. Herndon J, Hallemeier C, Jethwa K, Shariff A, Bancos I. Clin Endocrinol (Oxf) 2023;99:35–42. doi: 10.1111/cen.14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Management of endocrine disease: Cushing's syndrome due to ectopic ACTH secretion: an expert operational opinion. Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. Eur J Endocrinol. 2020;182:0–58. doi: 10.1530/EJE-19-0877. [DOI] [PubMed] [Google Scholar]
- 17.Clinical heterogeneity of ectopic ACTH syndrome: a long-term follow-up study. Espinosa-de-Los-Monteros AL, Ramírez-Rentería C, Mercado M. Endocr Pract. 2020;26:1435–1441. doi: 10.4158/EP-2020-0368. [DOI] [PubMed] [Google Scholar]
- 18.Characteristics and outcomes of the Finnish ectopic ACTH syndrome cohort. Toivanen S, Leijon H, Arola A, et al. Endocrine. 2021;74:387–395. doi: 10.1007/s12020-021-02768-0. [DOI] [PubMed] [Google Scholar]
- 19.SBRT to adrenal metastases provides high local control with minimal toxicity. Plichta K, Camden N, Furqan M, et al. Adv Radiat Oncol. 2017;2:581–587. doi: 10.1016/j.adro.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stereotactic body radiation therapy of adrenal metastases: a pooled meta-analysis and systematic review of 39 studies with 1006 patients. Chen WC, Baal JD, Baal U, et al. Int J Radiat Oncol Biol Phys. 2020;107:48–61. doi: 10.1016/j.ijrobp.2020.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]