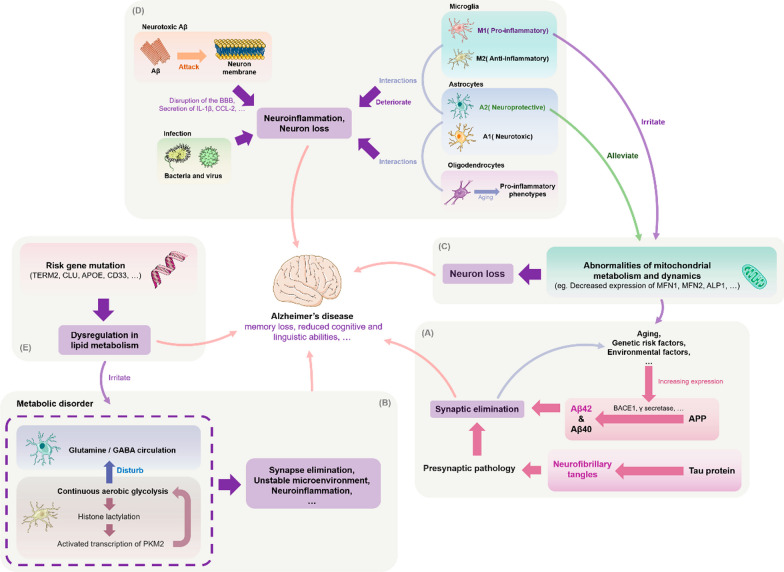
Fig. 1.
Possible pathogenesis of Alzheimer's disease. A Amyloid-beta precursor protein (APP) is cleaved by BACE1 and other enzymes in the amyloid pathogenic pathway to form different isoforms of Aβ (this process can also be affected by gene mutations), which rapidly aggregate to form neurotoxic oligomers that can induce synaptic loss and further cause cognitive impairment and dementia. Simultaneously, Aβ can contribute to tau pathology by increasing abnormal phosphorylation and the formation of toxic neurofibrillary tangles (NFTs), which can lead to neurodegeneration. According to the synaptic defect hypothesis, synaptic damage is the underlying cause of AD and defective synapses may lead to the formation of neurotoxic Aβ. B One potential mechanism of AD progression is dysregulation of multiple metabolic pathways. Microglia glucose metabolism disruption promotes a vicious cycle of "glycolysis-histone lactation-PKM2", leading to an imbalance of microglia homeostasis and neuroinflammation. Astrocyte metabolic failure also impairs the neurotransmitter glutamate/GABA glutamine cycle and causes synaptic dysfunction. C Dysfunctional mitochondrial dynamics can result in aberrant mitochondrial fusion fission, in turn causing synaptic degeneration and neuronal injury. Such dynamics include diminished expression of the proteins that control this process. By transferring healthy mitochondria, astrocytes could reduce the negative effects of damaged mitochondria on neurons. This function is blocked in chronic inflammatory situations, which may hasten AD progression. The removal of Aβ and emergence of tau disease may also be impacted by aberrant mitochondrial autophagy. D Microglia, astrocytes, and oligodendrocytes have pro-inflammatory phenotypes that promote neuroinflammation and the development of AD. The three types of glial cells interact with one another to speed up neuroinflammation. For example, activated microglia secrete IL-1a, TNF-a, and C1q, which cause reactive astrocytes to develop into the A1 neurotoxic phenotype, whereas oligodendrocytes cause astrocytes to develop into the NF-B signaling or A1 phenotype. Other harmful processes in AD include the attack of Aβ on its own neuronal cell membranes and bacterial-viral infection. E Mutations in common risk genes, including APOE, triggering receptor expressed on myeloid cells 2 (TREM2), CLU, and CD33, may disrupt normal functions—particularly lipid metabolism in glial cells—thereby increasing the risk of AD development