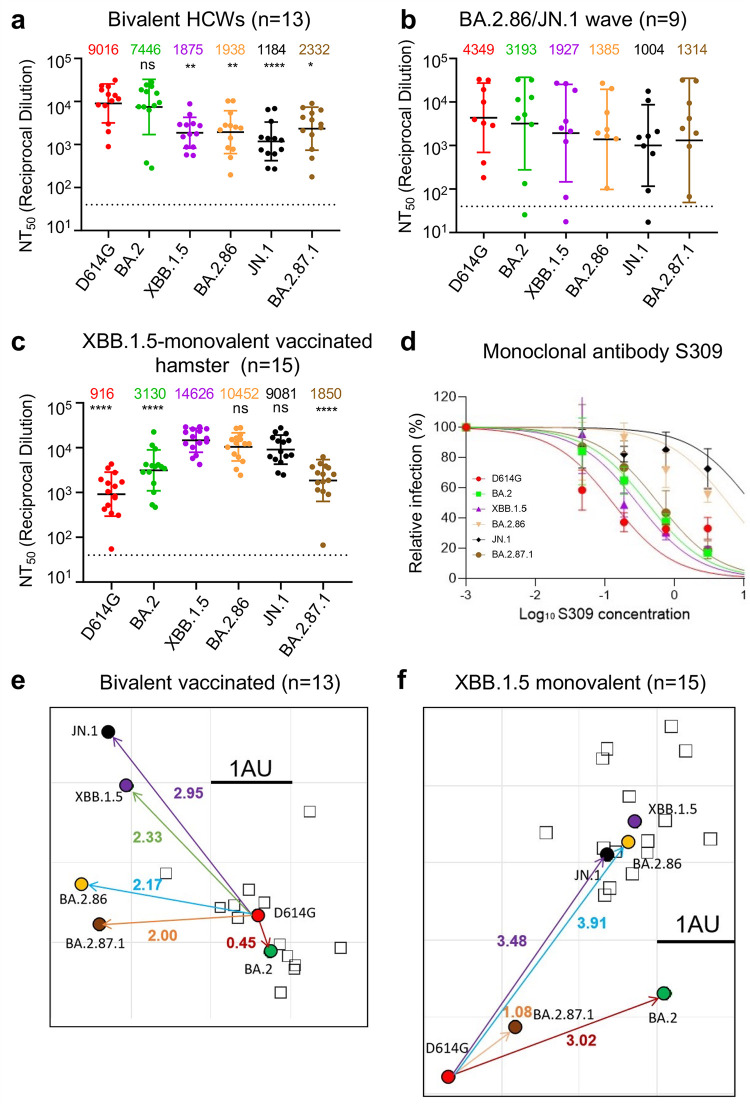
Fig 2.
Neutralization of BA.2.87.1 and JN.1 by bivalent-vaccinated human sera, JN.1-wave human sera, XBB.1.5-vaccinated hamster sera, and monoclonal antibody S309. (a–c) NAb titers were determined using lentiviruses bearing the indicated spike proteins, with the titer of D614G as a control. All were compared against D614G or XBB.1.5 unless otherwise specified. The three cohorts included sera from 13 HCWs who had at least 2 monovalent doses of mRNA vaccine and 1 dose of bivalent mRNA vaccine (n = 13) (a), sera from Columbus first-responder/household contact cohort (P1–P5) and ICU patients admitted to OSU Wexner Medical Center (P6–P9) during when the BA.2.86/JN.1 variants were predominantly circulating in Columbus, Ohio (b) (n = 9 total), and sera from Golden Syrian hamsters inoculated with two doses of XBB.1.5 monovalent vaccine (recombinant mumps virus expressing the spike of XBB.1.5, 1.5 × 105 PFU per hamster, 3 weeks apart) (n = 15), with blood being collected 5 weeks after inoculation (c). Geometric mean NT50 values for each variant are shown on the top. Bars represent geometric means with 95% confidence intervals. Statistical significance was analyzed with log10 transformed NT50 values. Comparisons between multiple groups were performed using a one-way ANOVA with Bonferroni post-test. Dashed lines represent the threshold of detection, i.e., NT50 = 40. P values are shown as ns P > 0.05, *P < 0.05, **P < 0.01, ****P < 0.0001. (d) Neutralization by mAb S309 was assessed, with representative plot curves displayed. Bars represent means ± standard deviation. (e and f) Antigenic maps for neutralization titers from Fig. 2a (bivalent-vaccinated human sera) and Fig. 2c (XBB.1.5-monovalent-vaccinated hamster sera) were made using the Racmacs program (1.1.35) (see Materials and Methods). Squares represent the individual sera sample and circles represent variants. One square on the grid represents one antigenic unit squared.