Fig 2.
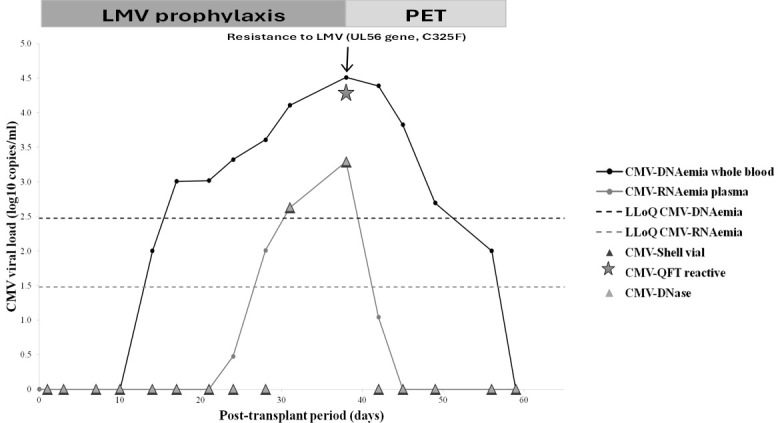
Virological monitoring by CMV-DNAemia and CMV-RNAemia in a hematopoietic stem cell transplant recipient managed by LMV prophylaxis during post-transplant. PET, pre-emptive therapy with foscarnet; LLoQ, lower limit of quantification; CMV-DNase, CMV-DNAemia detection in plasma samples after DNase digestion; CMV-QFT reactive, presence of CMV cell-mediated immune response measured by QuantiFERON-CMVhttps://www.qiagen.com/us/resources/download.aspx?id=42713b7e-2b8f-48b6-8198-44104d14aacb&lang=it-ITtest (QIAGEN). Adult hematopoietic stem cell transplant recipient receiving LMV prophylaxis. An episode of active viral replication was identified by positive results of CMV-RNAemia and of the additional routine methods (CMV-DNase and CMV-viremia). Pre-emptive therapy was started based on both clinical and laboratory findings, obtaining a complete viral clearance as shown by CMV-DNAemia and more rapidly by CMV-RNAemia.
